Effectiveness of COVID-19 booster vaccines against COVID-19-related symptoms, hospitalization and death in England
- PMID: 35045566
- PMCID: PMC9018410
- DOI: 10.1038/s41591-022-01699-1
Effectiveness of COVID-19 booster vaccines against COVID-19-related symptoms, hospitalization and death in England
Abstract
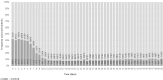
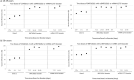
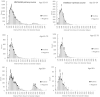
Booster vaccination with messenger RNA (mRNA) vaccines has been offered to adults in England starting on 14 September 2021. We used a test-negative case-control design to estimate the relative effectiveness of a booster dose of BNT162b2 (Pfizer-BioNTech) compared to only a two-dose primary course (at least 175 days after the second dose) or unvaccinated individuals from 13 September 2021 to 5 December 2021, when Delta variant was dominant in circulation. Outcomes were symptomatic coronavirus disease 2019 (COVID-19) and hospitalization. The relative effectiveness against symptomatic disease 14-34 days after a BNT162b2 or mRNA-1273 (Moderna) booster after a ChAdOx1-S (AstraZeneca) and BNT162b2 as a primary course ranged from around 85% to 95%. Absolute vaccine effectiveness ranged from 94% to 97% and was similar in all age groups. Limited waning was seen 10 or more weeks after the booster. Against hospitalization or death, absolute effectiveness of a BNT162b2 booster ranged from around 97% to 99% in all age groups irrespective of the primary course, with no evidence of waning up to 10 weeks. This study provides real-world evidence of substantially increased protection from the booster vaccine dose against mild and severe disease irrespective of the primary course.
© 2022. Crown.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Pritchard E, et al. Impact of vaccination on SARS-CoV-2 cases in the community: a population-based study using the UK’s COVID-19 infection survey. Nat. Med. 2021;27:370–1378. doi: 10.1038/s41591-021-01410-w. - DOI
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

