HCV Infection Alters Salivary Gland Histology and Saliva Composition
- PMID: 35045743
- PMCID: PMC9052835
- DOI: 10.1177/00220345211049395
HCV Infection Alters Salivary Gland Histology and Saliva Composition
Abstract
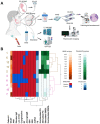
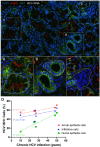
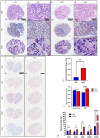
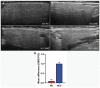
Hepatitis C virus (HCV) infection is the most common blood-borne chronic infection in the United States. Chronic lymphocytic sialadenitis and sicca syndrome have been reported in chronic HCV infection. Up to 55% of these patients may have xerostomia; the mechanisms of the xerostomia and salivary gland (SG) hypofunction remain controversial. The objectives of this project are to establish if xerostomia associates with SG and HCV infection and to characterize the structural changes in SG and saliva composition. Eighteen HCV-infected patients with xerostomia were evaluated for SG dysfunction; 6 of these patients (patients 1-6) were further evaluated for SG histopathological changes and changes in saliva composition. The techniques used include clinical and laboratory assessment, SG ultrasonography, histological evaluation, sialochemical and proteomics analysis, and RNA in situ hybridization. All the HCV patients had low saliva flow, chronic sialadenitis, and SG fibrosis and lacked Sjögren syndrome (SS) characteristic autoantibodies. Further evaluation of a subgroup of 6 HCV patients (patients 1-6) demonstrated diffuse lymphocytic infiltrates that are predominantly CD8+ T cells with a significant increase in the number of inflammatory cells. Alcian Blue/periodic acid-Schiff staining showed significant changes in the ratio and intensity of the acinar secretory units of the HCV patients' minor SG. The submandibular glands showed significant ultrasonographic abnormalities in the parenchyma relative to the parotid glands. Significant changes were also observed in the concentration of sodium and mucin 5b. Although no significant correlation was observed between the lymphocytic infiltrates and the years of HCV chronic infection, a positive correlation was observed between HCV RNA-positive epithelial cells and the years of HCV infection. Consistent with the low saliva flow and xerostomia, patients showed changes in several markers of SG acinar and ductal function. Changes in the composition of the saliva suggest that HCV infection can cause xerostomia by mechanisms distinct from SS.
Keywords: Sjögren’s syndrome; chronic infection; salivary hypofunction; sialadenitis; sicca syndrome; xerostomia.
Conflict of interest statement
Figures
References
-
- Aceti A, Taliani G, Sorice M, Amendolea MA. 1992. HCV and Sjögren’s syndrome. Lancet. 339(8806):1425–1426. - PubMed
-
- Alliende C, Kwon YJ, Brito M, Molina C, Aguilera S, Perez P, Leyton L, Quest AF, Mandel U, Veerman E, et al.. 2008. Reduced sulfation of MUC5B is linked to xerostomia in patients with Sjögren syndrome. Ann Rheum Dis. 67(10):1480–1487. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

