Patients with Rare Cancers in the Drug Rediscovery Protocol (DRUP) Benefit from Genomics-Guided Treatment
- PMID: 35046062
- PMCID: PMC9365364
- DOI: 10.1158/1078-0432.CCR-21-3752
Patients with Rare Cancers in the Drug Rediscovery Protocol (DRUP) Benefit from Genomics-Guided Treatment
Abstract
Purpose: Patients with rare cancers (incidence less than 6 cases per 100,000 persons per year) commonly have less treatment opportunities and are understudied at the level of genomic targets. We hypothesized that patients with rare cancer benefit from approved anticancer drugs outside their label similar to common cancers.
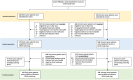
Experimental design: In the Drug Rediscovery Protocol (DRUP), patients with therapy-refractory metastatic cancers harboring an actionable molecular profile are matched to FDA/European Medicines Agency-approved targeted therapy or immunotherapy. Patients are enrolled in parallel cohorts based on the histologic tumor type, molecular profile and study drug. Primary endpoint is clinical benefit (complete response, partial response, stable disease ≥ 16 weeks).
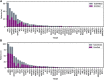
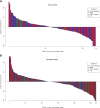
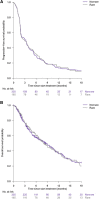
Results: Of 1,145 submitted cases, 500 patients, including 164 patients with rare cancers, started one of the 25 available drugs and were evaluable for treatment outcome. The overall clinical benefit rate was 33% in both the rare cancer and nonrare cancer subgroup. Inactivating alterations of CDKN2A and activating BRAF aberrations were overrepresented in patients with rare cancer compared with nonrare cancers, resulting in more matches to CDK4/6 inhibitors (14% vs. 4%; P ≤ 0.001) or BRAF inhibitors (9% vs. 1%; P ≤ 0.001). Patients with rare cancer treated with small-molecule inhibitors targeting BRAF experienced higher rates of clinical benefit (75%) than the nonrare cancer subgroup.
Conclusions: Comprehensive molecular testing in patients with rare cancers may identify treatment opportunities and clinical benefit similar to patients with common cancers. Our findings highlight the importance of access to broad molecular diagnostics to ensure equal treatment opportunities for all patients with cancer.
©2022 The Authors; Published by the American Association for Cancer Research.
Figures

References
-
- Gatta G, Capocaccia R, Botta L, Mallone S, De Angelis R, Ardanaz E, et al. . Burden and centralised treatment in Europe of rare tumours: results of RARECAREnet-a population-based study. Lancet Oncol 2017;18:1022–39. - PubMed
-
- Gatta G, van der Zwan JM, Casali PG, Siesling S, Dei Tos AP, Kunkler I, et al. . Rare cancers are not so rare: the rare cancer burden in Europe. Eur J Cancer 2011;47:2493–511. - PubMed
-
- Huang RSP, Haberberger J, Severson E, Duncan DL, Hemmerich A, Edgerly C, et al. . A pan-cancer analysis of PD-L1 immunohistochemistry and gene amplification, tumor mutation burden and microsatellite instability in 48,782 cases. Mod Pathol 2021;34:252–63. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

