KIFC1 Regulates the Trajectory of Neuronal Migration
- PMID: 35046122
- PMCID: PMC8936618
- DOI: 10.1523/JNEUROSCI.1708-21.2022
KIFC1 Regulates the Trajectory of Neuronal Migration
Abstract
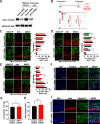
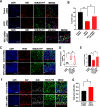
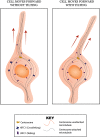
During neuronal migration, forces generated by cytoplasmic dynein yank on microtubules extending from the centrosome into the leading process and move the nucleus along microtubules that extend behind the centrosome. Scaffolds, such as radial glia, guide neuronal migration outward from the ventricles, but little is known about the internal machinery that ensures that the soma migrates along its proper path rather than moving backward or off the path. Here we report that depletion of KIFC1, a minus-end-directed kinesin called HSET in humans, causes neurons to migrate off their appropriate path, suggesting that this molecular motor is what ensures fidelity of the trajectory of migration. For these studies, we used rat migratory neurons in vitro and developing mouse brain in vivo, together with RNA interference and ectopic expression of mutant forms of KIFC1. We found that crosslinking of microtubules into a nonsliding mode by KIFC1 is necessary for dynein-driven forces to achieve sufficient traction to thrust the soma forward. Asymmetric bouts of microtubule sliding driven by KIFC1 thereby enable the soma to tilt in one direction or another, thus providing midcourse corrections that keep the neuron on its appropriate trajectory. KIFC1-driven sliding of microtubules further assists neurons in remaining on their appropriate path by allowing the nucleus to rotate directionally as it moves, which is consistent with how we found that KIFC1 contributes to interkinetic nuclear migration at an earlier stage of neuronal development.SIGNIFICANCE STATEMENT Resolving the mechanisms of neuronal migration is medically important because many developmental disorders of the brain involve flaws in neuronal migration and because deployment of newly born neurons may be important in the adult for cognition and memory. Drugs that inhibit KIFC1 are candidates for chemotherapy and therefore should be used with caution if they are allowed to enter the brain.
Keywords: HSET; KIFC1; cytoplasmic dynein; microtubule; molecular motor; neuronal migration.
Copyright © 2022 the authors.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
