Quantification of bone marrow interstitial pH and calcium concentration by intravital ratiometric imaging
- PMID: 35046411
- PMCID: PMC8770570
- DOI: 10.1038/s41467-022-27973-x
Quantification of bone marrow interstitial pH and calcium concentration by intravital ratiometric imaging
Erratum in
-
Publisher Correction: Quantification of bone marrow interstitial pH and calcium concentration by intravital ratiometric imaging.Nat Commun. 2022 Mar 17;13(1):1563. doi: 10.1038/s41467-022-28925-1. Nat Commun. 2022. PMID: 35302057 Free PMC article. No abstract available.
Abstract
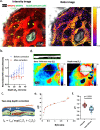
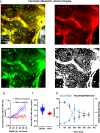
The fate of hematopoietic stem cells (HSCs) can be directed by microenvironmental factors including extracellular calcium ion concentration ([Ca2+]e), but the local [Ca2+]e around individual HSCs in vivo remains unknown. Here we develop intravital ratiometric analyses to quantify the absolute pH and [Ca2+]e in the mouse calvarial bone marrow, taking into account the pH sensitivity of the calcium probe and the wavelength-dependent optical loss through bone. Unexpectedly, the mean [Ca2+]e in the bone marrow (1.0 ± 0.54 mM) is not significantly different from the blood serum, but the HSCs are found in locations with elevated local [Ca2+]e (1.5 ± 0.57 mM). With aging, a significant increase in [Ca2+]e is found in M-type cavities that exclusively support clonal expansion of activated HSCs. This work thus establishes a tool to investigate [Ca2+]e and pH in the HSC niche with high spatial resolution and can be broadly applied to other tissue types.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

