Merging enzymes with chemocatalysis for amide bond synthesis
- PMID: 35046426
- PMCID: PMC8770729
- DOI: 10.1038/s41467-022-28005-4
Merging enzymes with chemocatalysis for amide bond synthesis
Abstract
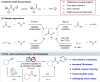
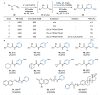
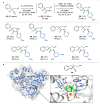
Amides are one of the most fundamental chemical bonds in nature. In addition to proteins and other metabolites, many valuable synthetic products comprise amide bonds. Despite this, there is a need for more sustainable amide synthesis. Herein, we report an integrated next generation multi-catalytic system, merging nitrile hydratase enzymes with a Cu-catalysed N-arylation reaction in a single reaction vessel, for the construction of ubiquitous amide bonds. This synergistic one-pot combination of chemo- and biocatalysis provides an amide bond disconnection to precursors, that are orthogonal to those in classical amide synthesis, obviating the need for protecting groups and delivering amides in a manner unachievable using existing catalytic regimes. Our integrated approach also affords broad scope, very high (molar) substrate loading, and has excellent functional group tolerance, telescoping routes to natural product derivatives, drug molecules, and challenging chiral amides under environmentally friendly conditions at scale.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Pattabiraman VR, Bode JW. Rethinking amide bond synthesis. Nature. 2011;480:471–479. - PubMed
-
- Boström J, Brown DG, Young RJ, Keserü GM. Expanding the medicinal chemistry synthetic toolbox. Nat. Rev. Drug Discov. 2018;17:922–922. - PubMed
-
- Sabatini MT, Boulton LT, Sneddon HF, Sheppard TD. A green chemistry perspective on catalytic amide bond formation. Nat. Catal. 2019;2:10–17.
-
- Constable DJC, et al. Key green chemistry research areas—a perspective from pharmaceutical manufacturers. Green Chem. 2007;9:411–420.
-
- Mahjour B, Shen Y, Liu W, Cernak T. A map of the amine–carboxylic acid coupling system. Nature. 2020;580:71–75. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

