Dose-related effects of ketamine for antidepressant-resistant symptoms of posttraumatic stress disorder in veterans and active duty military: a double-blind, randomized, placebo-controlled multi-center clinical trial
- PMID: 35046508
- PMCID: PMC8767037
- DOI: 10.1038/s41386-022-01266-9
Dose-related effects of ketamine for antidepressant-resistant symptoms of posttraumatic stress disorder in veterans and active duty military: a double-blind, randomized, placebo-controlled multi-center clinical trial
Erratum in
-
Correction to: Dose-related effects of ketamine for antidepressant-resistant symptoms of posttraumatic stress disorder in veterans and active duty military: a double-blind, randomized, placebo-controlled multi-center clinical trial.Neuropsychopharmacology. 2022 Jul;47(8):1583-1584. doi: 10.1038/s41386-022-01339-9. Neuropsychopharmacology. 2022. PMID: 35545665 Free PMC article. No abstract available.
Abstract
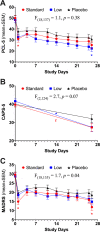
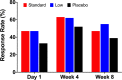
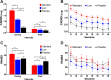
This study tested the efficacy of repeated intravenous ketamine doses to reduce symptoms of posttraumatic stress disorder (PTSD). Veterans and service members with PTSD (n = 158) who failed previous antidepressant treatment were randomized to 8 infusions administered twice weekly of intravenous placebo (n = 54), low dose (0.2 mg/kg; n = 53) or standard dose (0.5 mg/kg; n = 51) ketamine. Participants were assessed at baseline, during treatment, and for 4 weeks after their last infusion. Primary analyses used mixed effects models. The primary outcome measure was the self-report PTSD Checklist for DSM-5 (PCL-5), and secondary outcome measures were the Clinician-Administered PTSD Scale for DSM-5 (CAPS-5) and the Montgomery Åsberg Depression Rating Scale (MADRS). There were no significant group-by-time interactions for PTSD symptoms measured by the PCL-5 or CAPS-5. The standard ketamine dose ameliorated depression measured by the MADRS significantly more than placebo. Ketamine produced dose-related dissociative and psychotomimetic effects, which returned to baseline within 2 h and were less pronounced with repeated administration. There was no evidence of differential treatment discontinuation by ketamine dose, consistent with good tolerability. This clinical trial failed to find a significant dose-related effect of ketamine on PTSD symptoms. Secondary analyses suggested that the standard dose exerted rapid antidepressant effects. Further studies are needed to determine the role of ketamine in PTSD treatment. ClinicalTrials.gov identifier: NCT02655692.
© 2022. This is a U.S. government work and not under copyright protection in the U.S.; foreign copyright protection may apply.
Conflict of interest statement
Dr. Abdallah has served as a consultant, speaker and/or on advisory boards for Aptinyx, Genentech, Janssen, Psilocybin Labs, Lundbeck, Guidepoint, and FSV7, and as editor of Chronic Stress for Sage Publications, Inc. He also filed a patent for using mTORC1 inhibitors to augment the effects of antidepressants (Aug 20, 2018). Dr. Krystal is a consultant for Aptinyx, Inc., Atai Life Sciences, AstraZeneca Pharmaceuticals, Biogen, Idec, MA, Biomedisyn Corporation, Bionomics, Limited (Australia), Boehringer Ingelheim International, Cadent Therapeutics, Inc., Clexio Bioscience, Ltd., COMPASS Pathways, Limited, United Kingdom, Concert Pharmaceuticals, Inc., Epiodyne, Inc., EpiVario, Inc., Greenwich Biosciences, Inc., Heptares Therapeutics, Limited (UK), Janssen Research & Development, Jazz Pharmaceuticals, Inc., Otsuka America Pharmaceutical, Inc., Perception Neuroscience Holdings, Inc., Spring Care, Inc., Sunovion Pharmaceuticals, Inc., Takeda Industries, Taisho Pharmaceutical Co., Ltd. JHK also reports the following disclosures: Scientific Advisory Board: Biohaven Pharmaceuticals, BioXcel Therapeutics, Inc. (Clinical Advisory Board), Cadent Therapeutics, Inc. (Clinical Advisory Board), Cerevel Therapeutics, LLC, EpiVario, Inc., Eisai, Inc., Lohocla Research Corporation, Novartis Pharmaceuticals Corporation, PsychoGenics, Inc., RBNC Therapeutics, Inc., Tempero Bio, Inc., Terran Biosciences, Inc. Stock: Biohaven Pharmaceuticals, Sage Pharmaceuticals, Spring Care, Inc. Stock Options: Biohaven Pharmaceuticals Medical Sciences, EpiVario, Inc., RBNC Therapeutics, Inc., Terran Biosciences, Inc. Tempero Bio, Inc. Income Greater than $10,000: Editorial Board: Editor - Biological Psychiatry. Patents and Inventions: (1) Seibyl JP, Krystal JH, Charney DS Dopamine and noradrenergic reuptake inhibitors in treatment of schizophrenia. US Patent #:5,447,948.September 5, 1995. (2) Vladimir, Coric, Krystal, John H, Sanacora, Gerard – Glutamate Modulating Agents in the Treatment of Mental Disorders. US Patent No. 8,778,979 B2 Patent Issue Date: July 15, 2014. US Patent Application No. 15/695,164: Filing Date: 09/05/2017. (3) Charney D, Krystal JH, Manji H, Matthew S, Zarate C., - Intranasal Administration of Ketamine to Treat Depression United States Patent Number: 9592207, Issue date: 3/14/2017. Licensed to Janssen Research & Development. (4) Zarate, C, Charney, DS, Manji, HK, Mathew, Sanjay J, Krystal, JH, Yale University “Methods for Treating Suicidal Ideation”, Patent Application No. 15/379,013 filed on December 14, 2016 by Yale University Office of Cooperative Research. (5) Arias A, Petrakis I, Krystal JH. – Composition and methods to treat addiction. Provisional Use Patent Application no.61/973/961. April 2, 2014. Filed by Yale University Office of Cooperative Research. (6) Chekroud, A., Gueorguieva, R., & Krystal, JH. “Treatment Selection for Major Depressive Disorder” [filing date 3rd June 2016, USPTO docket number Y0087.70116US00]. Provisional patent submission by Yale University. (7) Gihyun, Yoon, Petrakis I, Krystal JH – Compounds, Compositions and Methods for Treating or Preventing Depression and Other Diseases. U. S. Provisional Patent Application No. 62/444,552, filed on January10, 2017 by Yale University Office of Cooperative Research OCR 7088 US01. (8) Abdallah, C, Krystal, JH, Duman, R, Sanacora, G. Combination Therapy for Treating or Preventing Depression or Other Mood Diseases. U.S. Provisional Patent Application No. 62/719,935 filed on August 20, 2018 by Yale University Office of Cooperative Research OCR 7451 US01. On Non-Federal Research Support: AstraZeneca Pharmaceuticals provides the drug, Saracatinib, for research related to NIAAA grant “Center for Translational Neuroscience of Alcoholism [CTNA-4] Novartis provides the drug, Mavoglurant, for research related to NIAAA grant “Center for Translational Neuroscience of Alcoholism [CTNA-4]. Dr. Gueorguieva discloses royalties from book “Statistical Methods in Psychiatry and Related Fields” published by CRC Press, honorarium as a member of the Working Group for PTSD Adaptive Platform Trial of Cohen Veterans Bioscience and a United States patent application 20200143922 by Yale University: Chekroud, A., Krystal, J., Gueorguieva, R. and Chandra, A. “Methods and Apparatus for Predicting Depression Treatment Outcomes”. Dr. Sherif is a consultant for In Silico Biosciences, Inc. Dr. Averill is a consultant for Guidepoint. All other co-authors declare no conflict of interest.
Figures
References
-
- Krystal JH, Davis LL, Neylan TC, M AR, Schnurr PP, Stein MB, et al. It is time to address the crisis in the pharmacotherapy of posttraumatic stress disorder: a consensus statement of the PTSD Psychopharmacology Working Group. Biol Psychiatry. 2017;82:e51–e9. doi: 10.1016/j.biopsych.2017.03.007. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

