Urine DNA biomarkers for hepatocellular carcinoma screening
- PMID: 35046521
- PMCID: PMC9091244
- DOI: 10.1038/s41416-022-01706-9
Urine DNA biomarkers for hepatocellular carcinoma screening
Abstract
Background: Hepatocellular carcinoma (HCC) occurs in a well-defined high-risk patient population, but better screening tests are needed to improve sensitivity and efficacy. Therefore, we investigated the use of urine circulating tumour DNA (ctDNA) as a screening test.
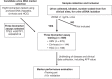
Methods: Candidate markers in urine were selected from HCC and controls. We then enrolled 609 patients from five medical centres to test the selected urine panel. A two-stage model was developed to combine AFP and urine panel as a screening test.
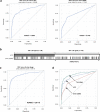
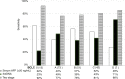
Results: Mutated TP53, and methylated RASSF1a, and GSTP1 were selected as the urine panel markers. Serum AFP outperformed the urine panel among all cases of HCC, but the urine panel identified 49% of HCC cases with low AFP < 20 ng/ml. Using the two-stage model, the combined AFP and urine panel identified 148 of the 186 HCC cases (79.6% sensitivity at 90% specificity), which was 30% more than the cases detected with serum AFP alone. It also increased early-stage HCC detection from 62% to 92% (BCLC stage 0), and 40% to 77% (BCLC stage A).
Conclusion: Urine ctDNA has promising diagnostic utility in patients in HCC, especially in those with low AFP and can be used as a potential non-invasive HCC screening test.
© 2022. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
AK is a consultant to AstraZeneca, WS, SJ and SL are shareholders of JBS Science Inc. YHS has received funding from JBS Science, Inc. The remaining authors declare no competing interests.
Figures

References
-
- Howlader N, Noone AM, Krapcho M, Miller D, Bishop K, Altekruse SF, et al. SEER cancer statistics review, 1975–2013, based on November 2015 SEER data submission, posted to the SEER website. Bethesda, MD, USA: National Cancer Institute; 2016.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

