The hospital exemption pathway for the approval of advanced therapy medicinal products: an underused opportunity? The case of the CAR-T ARI-0001
- PMID: 35046545
- PMCID: PMC8821008
- DOI: 10.1038/s41409-021-01463-y
The hospital exemption pathway for the approval of advanced therapy medicinal products: an underused opportunity? The case of the CAR-T ARI-0001
Abstract
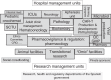
In February 2021, the 'Advanced Therapy Medicinal Product' (ATMP) ARI-0001 (CART19-BE-01), developed at Hospital Clínic de Barcelona (Spain), received authorization from the Spanish Agency of Medicines and Medical Devices (AEMPS) under the 'hospital exemption' (HE) approval pathway for the treatment of patients aged >25 years with relapsed/refractory (RR) acute lymphoblastic leukemia (ALL). The HE pathway foreseen by the European Regulation establishing the legal framework for ATMPs intended to be placed on the market in the EU, allows access to ATMPs prepared on a non-routine basis, according to quality standards, like a custom-made product for an individual patient. Its use is limited to the same Member State where it was developed, in a hospital under the responsibility of a medical practitioner. HE-ATMPs must comply with national traceability and pharmacovigilance requirements and specific quality standards. HE offers an opportunity to develop ATMPs in close contact with clinical practice, with the quality and rapid access needed by patients and at a lower cost compared to regular market authorization. However, many barriers need to be overcome. Here we discuss relevant aspects of the development and authorization of ARI-0001 in the context of the heterogeneous frame of the European Regulation implementation across the Member States.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- ARI-0001 Summary of Product Characteristics (Spanish). https://www.aemps.gob.es/investigacionClinica/terapiasAvanzadas/docs/ARI.... Accessed May 2021.
-
- Regulation (EC) No 1394/2007 of the European Parliament and of the Council of 13 November 2007 on advanced therapy medicinal products and amending Directive 2001/83/EC and Regulation (EC) No 726/200425th of February. https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2007:324:012.... Accessed May 2021.
-
- Ortiz-Maldonado V, Rives S, Castella M, Alonso-Saladrigues A, Benitez-Ribas D, Caballero-Banos M, et al. CART19-BE-01: a multicenter trial of ARI-0001 cell therapy in patients with CD19(+) relapsed/refractory malignancies. Mol Ther. 2021;29:636–44. doi: 10.1016/j.ymthe.2020.09.027. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

