Treatment Outcomes of High-Risk Non-Muscle Invasive Bladder Cancer (HR-NMIBC) in Real-World Evidence (RWE) Studies: Systematic Literature Review (SLR)
- PMID: 35046678
- PMCID: PMC8759992
- DOI: 10.2147/CEOR.S341896
Treatment Outcomes of High-Risk Non-Muscle Invasive Bladder Cancer (HR-NMIBC) in Real-World Evidence (RWE) Studies: Systematic Literature Review (SLR)
Abstract
Background: To date, there has been limited synthesis of RWE studies in high-risk non-muscle invasive bladder cancer (HR-NMIBC). The objective of this research was to conduct a systematic review of published real-world evidence to better understand the real-world burden and treatment patterns in HR-NMIBC.
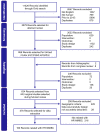
Methods: An SLR was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines with the scope defined by the Population, Intervention Comparators, Outcomes, and Study design (PICOS) criteria. EMBASE, MEDLINE, and Cochrane databases (Jan 2015-Jul 2020) were searched, and relevant congress abstracts (Jan 2018-Jul 2020) identified. The final analysis only included studies that enrolled ≥100 patients with HR-NMIBC from the US, Europe, Canada, and Australia.
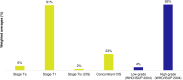
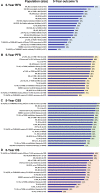
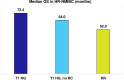
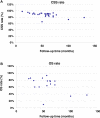
Results: The SLR identified 634 RWE publications in NMIBC, of which 160 studies reported data in HR-NMIBC. The average age of patients in the studies was 71 years, and 79% were males. The rates of BCG intravesical instillations ranged from 3% to 86% (29-95% for induction and 8-83% for maintenance treatment). Five-year outcomes were 17-89% recurrence-free survival (longest survival in patients completing BCG maintenance), 58-89% progression-free survival, 71-96% cancer-specific survival (lowest survival in BCG-unresponsive patients), and 28-90% overall survival (lowest survival in patients who did not receive BCG or instillation therapy).
Conclusion: BCG treatment rates and survival outcomes in patients with HR-NMIBC vary in the real world, with better survival seen in patients completing maintenance BCG, responding to treatment, and not progressing to muscle-invasive disease. There is a need to better understand the factors associated with BCG use and discontinuation and for an effective treatment that improves outcomes in HR-NMIBC. Generalization of these results is limited by variations in data collection, reporting, and methodologies used across RWE studies.
Keywords: high-risk NMIBC; real-world outcomes.
© 2022 Musat et al.
Conflict of interest statement
The following authors were Pfizer employees at the time the study was conducted: Elizabeth Masters and Slaven Sikirica. Debduth B Pijush was an employee of Atrium Staffing, which was a paid contractor to Pfizer in connection with the development of this manuscript. Mihaela Georgiana Musat, Christina Soeun Kwon, and Anna Forsythe are employees of Purple Squirrel Economics, which was a paid consultant to Pfizer in connection with the development of this manuscript. The authors report no other conflicts of interest in this work.
Figures
References
-
- National Cancer Institute. Cancer of the urinary bladder - Cancer stat facts. SEER; 2020. Available from: https://seer.cancer.gov/statfacts/html/urinb.html. Accessed September 28, 2020.
-
- NCCN. Bladder cancer, Version 6.2020, NCCN clinical practice guidelines in oncology. JNCCN; 2020. Available from: https://www.nccn.org/professionals/physician_gls/pdf/bladder_blocks.pdf. Accessed December 21, 2021.
-
- Babjuk M, Burger M, Comperat E, et al. EAU guidelines on non-muscle-invasive bladder cancer (TaT1 and CIS); 2020:1–54.
Publication types
LinkOut - more resources
Full Text Sources

