Immune Checkpoint Inhibitor-Induced Cerebral Pseudoprogression: Patterns and Categorization
- PMID: 35046955
- PMCID: PMC8761630
- DOI: 10.3389/fimmu.2021.798811
Immune Checkpoint Inhibitor-Induced Cerebral Pseudoprogression: Patterns and Categorization
Abstract
Background: The inclusion of immune checkpoint inhibitors (ICIs) in therapeutic algorithms has led to significant survival benefits in patients with various metastatic cancers. Concurrently, an increasing number of neurological immune related adverse events (IRAE) has been observed. In this retrospective analysis, we examine the ICI-induced incidence of cerebral pseudoprogression and propose a classification system.
Methods: We screened our hospital information system to identify patients with any in-house ICI treatment for any tumor disease during the years 2007-2019. All patients with cerebral MR imaging (cMRI) of sufficient diagnostic quality were included. cMRIs were retrospectively analyzed according to immunotherapy response assessment for neuro-oncology (iRANO) criteria.
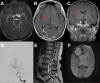
Results: We identified 12 cases of cerebral pseudoprogression in 123 patients treated with ICIs and sufficient MRI. These patients were receiving ICI therapy for lung cancer (n=5), malignant melanoma (n=4), glioblastoma (n=1), hepatocellular carcinoma (n=1) or lymphoma (n=1) when cerebral pseudoprogression was detected. Median time from the start of ICI treatment to pseudoprogression was 5 months. All but one patient developed neurological symptoms. Three different patterns of cerebral pseudoprogression could be distinguished: new or increasing contrast-enhancing lesions, new or increasing T2 predominant lesions and cerebral vasculitis type pattern.
Conclusion: Cerebral pseudoprogression followed three distinct patterns and was detectable in 3.2% of all patients during ICI treatment and in 9.75% of the patients with sufficient brain imaging follow up. The fact that all but one of the affected patients developed neurological symptoms, which would be classified as progressive disease according to iRANO criteria, mandates vigilance in the diagnosis and treatment of ICI-induced cerebral lesions.
Keywords: brain metastases; cerebral pseudoprogression; immune checkpoint inhibitors (ICI); immune related adverse events (irAE); immunotherapy; neurological complication; neurological side effects.
Copyright © 2022 Urban, Steidl, Hattingen, Filipski, Meissner, Sebastian, Koch, Strzelczyk, Forster, Baumgarten, Ronellenfitsch, Steinbach and Voss.
Conflict of interest statement
PB received travel grants from Roche and Zimmer Biomet. AS reports honoraria or research funding from Arvelle Therapeutics, Desitin Arzneimittel, Eisai, GW Pharmaceuticals companies, Marinus Pharmaceuticals, UCB, UNEEG medical, and Zogenix. JS has received honoraria for lectures or advisory board participation or consulting or travel grants from Abbvie, Roche, Novocure, Medac, Med-Update and UCB. MR has received a research grant from UCB. MS has received grants and personal fees from Roche, BMS, and AstraZeneca; personal fees from Abbvie, Takeda, MSD, Pfizer, Boehringer Ingelheim, Celgene, Biontech, CureVac, Novartis, Janssen, and Tesaro. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

