Effectiveness of Light-Quality and Dark-White Growth Light Shifts in Short-Term Light Acclimation of Photosynthesis in Arabidopsis
- PMID: 35046964
- PMCID: PMC8761940
- DOI: 10.3389/fpls.2021.615253
Effectiveness of Light-Quality and Dark-White Growth Light Shifts in Short-Term Light Acclimation of Photosynthesis in Arabidopsis
Abstract
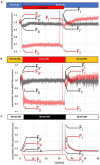
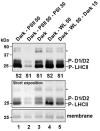
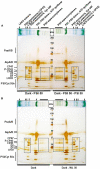
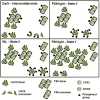
Photosynthesis needs to run efficiently under permanently changing illumination. To achieve this, highly dynamic acclimation processes optimize photosynthetic performance under a variety of rapidly changing light conditions. Such acclimation responses are acting by a complex interplay of reversible molecular changes in the photosynthetic antenna or photosystem assemblies which dissipate excess energy and balance uneven excitation between the two photosystems. This includes a number of non-photochemical quenching processes including state transitions and photosystem II remodeling. In the laboratory such processes are typically studied by selective illumination set-ups. Two set-ups known to be effective in a highly similar manner are (i) light quality shifts (inducing a preferential excitation of one photosystem over the other) or (ii) dark-light shifts (inducing a general off-on switch of the light harvesting machinery). Both set-ups result in similar effects on the plastoquinone redox state, but their equivalence in induction of photosynthetic acclimation responses remained still open. Here, we present a comparative study in which dark-light and light-quality shifts were applied to samples of the same growth batches of plants. Both illumination set-ups caused comparable effects on the phosphorylation of LHCII complexes and, hence, on the performance of state transitions, but generated different effects on the degree of state transitions and the formation of PSII super-complexes. The two light set-ups, thus, are not fully equivalent in their physiological effectiveness potentially leading to different conclusions in mechanistic models of photosynthetic acclimation. Studies on the regulation of photosynthetic light acclimation, therefore, requires to regard the respective illumination test set-up as a critical parameter that needs to be considered in the discussion of mechanistic and regulatory aspects in this subject.
Keywords: dark-light shifts; light-quality control; photosynthesis; photosystem II super-complexes; state transitions.
Copyright © 2022 Hommel, Liebers, Offermann and Pfannschmidt.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Allen J. F. (1992). Protein phosphorylation in regulation of photosynthesis. Biochim. Biophys. Acta 1098 275–335. - PubMed
LinkOut - more resources
Full Text Sources

