Efficient and regioselective synthesis of dihydroxy-substituted 2-aminocyclooctane-1-carboxylic acid and its bicyclic derivatives
- PMID: 35047084
- PMCID: PMC8744459
- DOI: 10.3762/bjoc.18.7
Efficient and regioselective synthesis of dihydroxy-substituted 2-aminocyclooctane-1-carboxylic acid and its bicyclic derivatives
Abstract
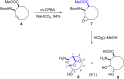
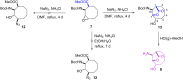
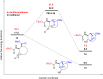
The first synthesis of 2-amino-3,4-dihydroxycyclooctane-1-carboxylic acid, methyl 6-hydroxy-9-oxo-8-oxabicyclo[5.2.1]decan-10-yl)carbamate, and 10-amino-6-hydroxy-8-oxabicyclo[5.2.1]decan-9-one starting from cis-9-azabicyclo[6.2.0]dec-6-en-10-one is described. cis-9-Azabicyclo[6.2.0]dec-6-en-10-one was transformed into the corresponding amino ester and its protected amine. Oxidation of the double bond in the N-Boc-protected methyl 2-aminocyclooct-3-ene-1-carboxylate then delivered the targeted amino acid and its derivatives. Density-functional theory (DFT) computations were used to explain the reaction mechanism for the ring opening of the epoxide and the formation of five-membered lactones. The stereochemistry of the synthesized compounds was determined by 1D and 2D NMR spectroscopy. The configuration of methyl 6-hydroxy-9-oxo-8-oxabicyclo[5.2.1]decan-10-yl)carbamate was confirmed by X-ray diffraction.
Keywords: DFT; aminocyclitol; azidolysis; bicyclic lactone; bicyclic β-lactam; cyclic β-amino acids.
Copyright © 2022, Polat et al.
Figures


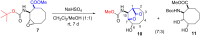




References
-
- von Nussbaum F, Spiteller P. β-Amino Acids in Nature. In: Schmuck C, Wennemers H, editors. Highlights in Bioorganic Chemistry: Methods and Applications. Weinheim, Germany: Wiley-VCH; 2004. pp. 63–89. - DOI
-
- Juaristi E, Soloshonok V. Enantioselective Synthesis of β-Amino Acids. 2nd ed. Hoboken, NJ, USA: John Wiley & Sons; 2005. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
