Location, location, location: subcellular protein partitioning in proteostasis and aging
- PMID: 35047088
- PMCID: PMC8724496
- DOI: 10.1007/s12551-021-00890-x
Location, location, location: subcellular protein partitioning in proteostasis and aging
Abstract
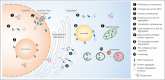
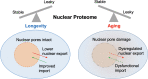
Somatic maintenance and cell survival rely on proper protein homeostasis to ensure reliable functions across the cell and to prevent proteome collapse. Maintaining protein folding and solubility is central to proteostasis and is coordinated by protein synthesis, chaperoning, and degradation capacities. An emerging aspect that influences proteostasis is the dynamic protein partitioning across different subcellular structures and compartments. Here, we review recent literature related to nucleocytoplasmic partitioning of proteins, nuclear and cytoplasmic quality control mechanisms, and their impact on the development of age-related diseases. We also highlight new points of entry to modulate spatially-regulated proteostatic mechanisms to delay aging.
Keywords: C. elegans; Nucleocytoplasmic partitioning; Proteostasis.
© The Author(s) 2021.
Conflict of interest statement
Conflict of interestThe authors declare no competing interests.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources