Understanding the Effects of Antipsychotics on Appetite Control
- PMID: 35047549
- PMCID: PMC8762106
- DOI: 10.3389/fnut.2021.815456
Understanding the Effects of Antipsychotics on Appetite Control
Abstract
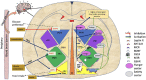
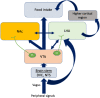
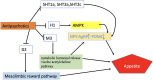
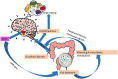
Antipsychotic drugs (APDs) represent a cornerstone in the treatment of schizophrenia and other psychoses. The effectiveness of the first generation (typical) APDs are hampered by so-called extrapyramidal side effects, and they have gradually been replaced by second (atypical) and third-generation APDs, with less extrapyramidal side effects and, in some cases, improved efficacy. However, the use of many of the current APDs has been limited due to their propensity to stimulate appetite, weight gain, and increased risk for developing type 2 diabetes and cardiovascular disease in this patient group. The mechanisms behind the appetite-stimulating effects of the various APDs are not fully elucidated, partly because their diverse receptor binding profiles may affect different downstream pathways. It is critical to identify the molecular mechanisms underlying drug-induced hyperphagia, both because this may lead to the development of new APDs, with lower appetite-stimulating effects but also because such insight may provide new knowledge about appetite regulation in general. Hence, in this review, we discuss the receptor binding profile of various APDs in relation to the potential mechanisms by which they affect appetite.
Keywords: antipsychotics; appetite control; hyperphagia; hypothalamus; mechanisms.
Copyright © 2022 Mukherjee, Skrede, Milbank, Andriantsitohaina, López and Fernø.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Nordentoft M, Wahlbeck K, Hallgren J, Westman J, Osby U, Alinaghizadeh H, et al. . Excess mortality, causes of death and life expectancy in 270,770 patients with recent onset of mental disorders in Denmark, Finland and Sweden. PLoS ONE. (2013) 8:e55176. 10.1371/journal.pone.0055176 - DOI - PMC - PubMed
-
- Liu NH, Daumit GL, Dua T, Aquila R, Charlson F, Cuijpers P, et al. . Excess mortality in persons with severe mental disorders: a multilevel intervention framework and priorities for clinical practice, policy and research agendas. World Psychiatry. (2017) 16:30–40. 10.1002/wps.20384 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

