Death With Function and Graft Failure After Kidney Transplantation: Risk Factors at Baseline Suggest New Approaches to Management
- PMID: 35047660
- PMCID: PMC8759617
- DOI: 10.1097/TXD.0000000000001273
Death With Function and Graft Failure After Kidney Transplantation: Risk Factors at Baseline Suggest New Approaches to Management
Abstract
Background: Improving both patient and graft survival after kidney transplantation are major unmet needs. The goal of this study was to assess risk factors for specific causes of graft loss to determine to what extent patients who develop either death with a functioning graft (DWFG) or graft failure (GF) have similar baseline risk factors for graft loss.
Methods: We retrospectively studied all solitary renal transplants performed between January 1, 2006, and December 31, 2018, at 3 centers and determined the specific causes of DWFG and GF. We examined outcomes in different subgroups using competing risk estimates and cause-specific Cox models.
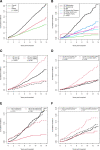
Results: Of the 5752 kidney transplants, graft loss occurred in 21.6% (1244) patients, including 12.0% (691) DWFG and 9.6% (553) GF. DWFG was most commonly due to malignancy (20.0%), infection (19.7%), cardiac disease (12.6%) with risk factors of older age and pretransplant dialysis, and diabetes as the cause of renal failure. For GF, alloimmunity (38.7%), glomerular diseases (18.6%), and tubular injury (13.9%) were the major causes. Competing risk incidence models identified diabetes and older recipients with higher rates of both DWFG and nonalloimmune GF.
Conclusions: These data suggest that at baseline, 2 distinct populations can be identified who are at high risk for renal allograft loss: a younger, nondiabetic patient group who develops GF due to alloimmunity and an older, more commonly diabetic population who develops DWFG and GF due to a mixture of causes-many nonalloimmune. Individualized management is needed to improve long-term renal allograft survival in the latter group.
Copyright © 2022 The Author(s). Transplantation Direct. Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
The authors declare no funding or conflicts of interest.
Figures



References
-
- Lamb KE, Lodhi S, Meier-Kriesche HU. Long-term renal allograft survival in the United States: a critical reappraisal. Am J Transplant. 2011;11:450–462. - PubMed
-
- Verduijn M, Grootendorst DC, Dekker FW, et al. The analysis of competing events like cause-specific mortality–beware of the Kaplan-Meier method. Nephrol Dial Transplant. 2011;26:56–61. - PubMed
-
- Noordzij M, Leffondré K, van Stralen KJ, et al. When do we need competing risks methods for survival analysis in nephrology? Nephrol Dial Transplant. 2013;28:2670–2677. - PubMed
-
- El Ters M, Smith BH, Cosio FG, et al. Competing risk analysis in renal allograft survival: a new perspective to an old problem. Transplantation. 2021;105:668–676. - PubMed
-
- Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154.
LinkOut - more resources
Full Text Sources

