Publication rate in preclinical research: a plea for preregistration
- PMID: 35047690
- PMCID: PMC8647586
- DOI: 10.1136/bmjos-2019-100051
Publication rate in preclinical research: a plea for preregistration
Abstract
Objectives: The ultimate goal of biomedical research is the development of new treatment options for patients. Animal models are used if questions cannot be addressed otherwise. Currently, it is widely believed that a large fraction of performed studies are never published, but there are no data that directly address this question.
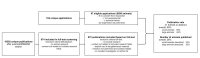
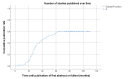
Methods: We have tracked a selection of animal study protocols approved in the University Medical Center Utrecht in the Netherlands, to assess whether these have led to a publication with a follow-up period of 7 years.
Results: We found that 60% of all animal study protocols led to at least one publication (full text or abstract). A total of 5590 animals were used in these studies, of which 26% was reported in the resulting publications.
Conclusions: The data presented here underline the need for preclinical preregistration, in view of the risk of reporting and publication bias in preclinical research. We plea that all animal study protocols should be prospectively registered on an online, accessible platform to increase transparency and data sharing. To facilitate this, we have developed a platform dedicated to animal study protocol registration: www.preclinicaltrials.eu.
Keywords: preclinicaltrials.eu; preregistration; publication bias; publication rate; translational research.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
Similar articles
-
A 3-year evaluation of preclinicaltrials.eu reveals room for improvement in preregistration of animal studies.PLoS Biol. 2021 Sep 9;19(9):e3001397. doi: 10.1371/journal.pbio.3001397. eCollection 2021 Sep. PLoS Biol. 2021. PMID: 34499640 Free PMC article.
-
Preregistration of animal research protocols: development and 3-year overview of preclinicaltrials.eu.BMJ Open Sci. 2022 Mar 16;6(1):e100259. doi: 10.1136/bmjos-2021-100259. eCollection 2022. BMJ Open Sci. 2022. PMID: 35372701 Free PMC article. Review.
-
Declaration of common standards for the preregistration of animal research-speeding up the scientific progress.PNAS Nexus. 2022 Mar 16;1(1):pgac016. doi: 10.1093/pnasnexus/pgac016. eCollection 2022 Mar. PNAS Nexus. 2022. PMID: 36712788 Free PMC article.
-
The future of Cochrane Neonatal.Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834
-
Publication Bias and Selective Outcome Reporting in Randomized Controlled Trials Related to Rehabilitation: A Literature Review.Arch Phys Med Rehabil. 2024 Jan;105(1):150-156. doi: 10.1016/j.apmr.2023.06.006. Epub 2023 Jun 25. Arch Phys Med Rehabil. 2024. PMID: 37364686 Review.
Cited by
-
Investigating the replicability of preclinical cancer biology.Elife. 2021 Dec 7;10:e71601. doi: 10.7554/eLife.71601. Elife. 2021. PMID: 34874005 Free PMC article.
-
A 3-year evaluation of preclinicaltrials.eu reveals room for improvement in preregistration of animal studies.PLoS Biol. 2021 Sep 9;19(9):e3001397. doi: 10.1371/journal.pbio.3001397. eCollection 2021 Sep. PLoS Biol. 2021. PMID: 34499640 Free PMC article.
-
Repurposing medications.Ocul Surf. 2021 Jan;19:336-340. doi: 10.1016/j.jtos.2020.11.012. Epub 2020 Nov 26. Ocul Surf. 2021. PMID: 33249292 Free PMC article. No abstract available.
-
The PERMIT guidelines for designing and implementing all stages of personalised medicine research.Sci Rep. 2024 Nov 13;14(1):27894. doi: 10.1038/s41598-024-79161-0. Sci Rep. 2024. PMID: 39537728 Free PMC article.
-
Targeting Impaired Nutrient Sensing via the Glycogen Synthase Kinase-3 Pathway With Therapeutic Compounds to Prevent or Treat Dementia: A Systematic Review.Front Aging. 2022 Jul 18;3:898853. doi: 10.3389/fragi.2022.898853. eCollection 2022. Front Aging. 2022. PMID: 35923682 Free PMC article. Review.
References
-
- Chalmers I, Glasziou P, Chalmers I, et al. . Research waste is still a scandal — an essay by Paul 2018;4645:10–12.
LinkOut - more resources
Full Text Sources
