In vitro Studies of Transendothelial Migration for Biological and Drug Discovery
- PMID: 35047883
- PMCID: PMC8757899
- DOI: 10.3389/fmedt.2020.600616
In vitro Studies of Transendothelial Migration for Biological and Drug Discovery
Abstract
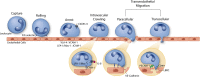
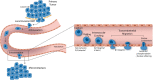
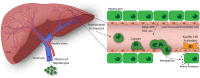
Inflammatory diseases and cancer metastases lack concrete pharmaceuticals for their effective treatment despite great strides in advancing our understanding of disease progression. One feature of these disease pathogeneses that remains to be fully explored, both biologically and pharmaceutically, is the passage of cancer and immune cells from the blood to the underlying tissue in the process of extravasation. Regardless of migratory cell type, all steps in extravasation involve molecular interactions that serve as a rich landscape of targets for pharmaceutical inhibition or promotion. Transendothelial migration (TEM), or the migration of the cell through the vascular endothelium, is a particularly promising area of interest as it constitutes the final and most involved step in the extravasation cascade. While in vivo models of cancer metastasis and inflammatory diseases have contributed to our current understanding of TEM, the knowledge surrounding this phenomenon would be significantly lacking without the use of in vitro platforms. In addition to the ease of use, low cost, and high controllability, in vitro platforms permit the use of human cell lines to represent certain features of disease pathology better, as seen in the clinic. These benefits over traditional pre-clinical models for efficacy and toxicity testing are especially important in the modern pursuit of novel drug candidates. Here, we review the cellular and molecular events involved in leukocyte and cancer cell extravasation, with a keen focus on TEM, as discovered by seminal and progressive in vitro platforms. In vitro studies of TEM, specifically, showcase the great experimental progress at the lab bench and highlight the historical success of in vitro platforms for biological discovery. This success shows the potential for applying these platforms for pharmaceutical compound screening. In addition to immune and cancer cell TEM, we discuss the promise of hepatocyte transplantation, a process in which systemically delivered hepatocytes must transmigrate across the liver sinusoidal endothelium to successfully engraft and restore liver function. Lastly, we concisely summarize the evolving field of porous membranes for the study of TEM.
Keywords: drug discovery; extravasation; hepatocyte transplantation; in vitro platforms; leukocytes; metastasis; porous membranes; transendothelial migration.
Copyright © 2020 Salminen, Allahyari, Gholizadeh, McCloskey, Ajalik, Cottle, Gaborski and McGrath.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

