Evolution of the Vagus Nerve Stimulation (VNS) Therapy System Technology for Drug-Resistant Epilepsy
- PMID: 35047938
- PMCID: PMC8757869
- DOI: 10.3389/fmedt.2021.696543
Evolution of the Vagus Nerve Stimulation (VNS) Therapy System Technology for Drug-Resistant Epilepsy
Abstract
The vagus nerve stimulation (VNS) Therapy® System is the first FDA-approved medical device therapy for the treatment of drug-resistant epilepsy. Over the past two decades, the technology has evolved through multiple iterations resulting in software-related updates and implantable lead and generator hardware improvements. Healthcare providers today commonly encounter a range of single- and dual-pin generators (models 100, 101, 102, 102R, 103, 104, 105, 106, 1000) and related programming systems (models 250, 3000), all of which have their own subtle, but practical differences. It can therefore be a daunting task to go through the manuals of these implant models for comparison, some of which are not readily available. In this review, we highlight the technological evolution of the VNS Therapy System with respect to device approval milestones and provide a comparison of conventional open-loop vs. the latest closed-loop generator models. Battery longevity projections and an in-depth examination of stimulation mode interactions are also presented to further differentiate amongst generator models.
Keywords: VNS; drug-resistant epilepsy; medical device; neuromodulation; vagus nerve stimulation.
Copyright © 2021 Afra, Adamolekun, Aydemir and Watson.
Conflict of interest statement
GW is an employee of LivaNova. PA and SA have participated in clinical trials for LivaNova. The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

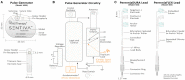


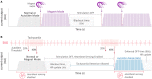
References
-
- LivaNova Inc . VNS Therapy Physician's Manual. LivaNova, Inc., 26-0009-7600/6 (U.S.) (2020).
-
- Arle JE. New vagus nerve stimulation lead and implantable pulse generator placement. In: Arle JE, editor, The Neuromodulation Casejournal. Cambridge, MA, Academic Press; (2020). p. 153–8. 10.1016/B978-0-12-817002-1.00020-1 - DOI
-
- LivaNova Inc . MRI with the VNS Therapy® System. LivaNova, Inc., 26-0010-0300/2 (worldwide) (2019).
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

