Excessive osteoclast activation by osteoblast paracrine factor RANKL is a major cause of the abnormal long bone phenotype in Apert syndrome model mice
- PMID: 35048384
- PMCID: PMC9303724
- DOI: 10.1002/jcp.30682
Excessive osteoclast activation by osteoblast paracrine factor RANKL is a major cause of the abnormal long bone phenotype in Apert syndrome model mice
Abstract
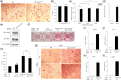
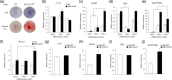
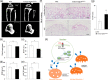
The fibroblast growth factor (FGF)/FGF receptor (FGFR) signaling pathway plays important roles in the development and growth of the skeleton. Apert syndrome caused by gain-of-function mutations of FGFR2 results in aberrant phenotypes of the skull, midface, and limbs. Although short limbs are representative features in patients with Apert syndrome, the causative mechanism for this limb defect has not been elucidated. Here we quantitatively confirmed decreases in the bone length, bone mineral density, and bone thickness in the Apert syndrome model of gene knock-in Fgfr2S252W/+ (EIIA-Fgfr2S252W/+ ) mice. Interestingly, despite these bone defects, histological analysis showed that the endochondral ossification process in the mutant mice was similar to that in wild-type mice. Tartrate-resistant acid phosphatase staining revealed that trabecular bone loss in mutant mice was associated with excessive osteoclast activity despite accelerated osteogenic differentiation. We investigated the osteoblast-osteoclast interaction and found that the increase in osteoclast activity was due to an increase in the Rankl level of osteoblasts in mutant mice and not enhanced osteoclastogenesis driven by the activation of FGFR2 signaling in bone marrow-derived macrophages. Consistently, Col1a1-Fgfr2S252W/+ mice, which had osteoblast-specific expression of Fgfr2 S252W, showed significant bone loss with a reduction of the bone length and excessive activity of osteoclasts was observed in the mutant mice. Taken together, the present study demonstrates that the imbalance in osteoblast and osteoclast coupling by abnormally increased Rankl expression in Fgfr2S252W/+ mutant osteoblasts is a major causative mechanism for bone loss and short long bones in Fgfr2S252W/+ mice.
Keywords: Apert syndrome; FGFR2; Rankl; long bone; osteoblast-osteoclast interaction.
© 2022 The Authors. Journal of Cellular Physiology published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare that they have no conflict of interests.
Figures



Similar articles
-
The Fgfr2(S252W/+) mutation in mice retards mandible formation and reduces bone mass as in human Apert syndrome.Am J Med Genet A. 2013 May;161A(5):983-92. doi: 10.1002/ajmg.a.35824. Epub 2013 Mar 13. Am J Med Genet A. 2013. PMID: 23495007
-
Apert syndrome mutant FGFR2 and its soluble form reciprocally alter osteogenesis of primary calvarial osteoblasts.J Cell Physiol. 2012 Sep;227(9):3267-77. doi: 10.1002/jcp.24021. J Cell Physiol. 2012. PMID: 22105374
-
A Ser252Trp mutation in fibroblast growth factor receptor 2 (FGFR2) mimicking human Apert syndrome reveals an essential role for FGF signaling in the regulation of endochondral bone formation.PLoS One. 2014 Jan 28;9(1):e87311. doi: 10.1371/journal.pone.0087311. eCollection 2014. PLoS One. 2014. PMID: 24489893 Free PMC article.
-
Osteoclast differentiation by RANKL and OPG signaling pathways.J Bone Miner Metab. 2021 Jan;39(1):19-26. doi: 10.1007/s00774-020-01162-6. Epub 2020 Oct 20. J Bone Miner Metab. 2021. PMID: 33079279 Review.
-
Cleft Palate in Apert Syndrome.J Dev Biol. 2022 Aug 11;10(3):33. doi: 10.3390/jdb10030033. J Dev Biol. 2022. PMID: 35997397 Free PMC article. Review.
Cited by
-
Bone-targeted lipoplex-loaded three-dimensional bioprinting bilayer scaffold enhanced bone regeneration.Regen Biomater. 2024 Jun 3;11:rbae055. doi: 10.1093/rb/rbae055. eCollection 2024. Regen Biomater. 2024. PMID: 38867890 Free PMC article.
-
Fibroblast Insights into the Pathogenesis of Ankylosing Spondylitis.J Inflamm Res. 2023 Dec 22;16:6301-6317. doi: 10.2147/JIR.S439604. eCollection 2023. J Inflamm Res. 2023. PMID: 38149115 Free PMC article. Review.
-
Apert syndrome in a newborn with premature fusion of skull bones, a rostral nose, and cleft palate: A case report.Clin Case Rep. 2024 Aug 15;12(8):e9298. doi: 10.1002/ccr3.9298. eCollection 2024 Aug. Clin Case Rep. 2024. PMID: 39156200 Free PMC article.
-
Overexpression of fibroblast growth factor receptor 2 in bone marrow mesenchymal stem cells enhances osteogenesis and promotes critical cranial bone defect regeneration.Front Cell Dev Biol. 2023 May 17;11:1208239. doi: 10.3389/fcell.2023.1208239. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37266455 Free PMC article.
-
Nicotinamide enhances osteoblast differentiation through activation of the mitochondrial antioxidant defense system.Exp Mol Med. 2023 Jul;55(7):1531-1543. doi: 10.1038/s12276-023-01041-w. Epub 2023 Jul 18. Exp Mol Med. 2023. PMID: 37464093 Free PMC article.
References
-
- Baek, W. Y. , Lee, M. A. , Jung, J. W. , Kim, S. Y. , Akiyama, H. , de Crombrugghe, B. , & Kim, J. E. (2009). Positive regulation of adult bone formation by osteoblast‐specific transcription factor osterix. Journal of Bone and Mineral Research, 24(6), 1055–1065. 10.1359/jbmr.081248 - DOI - PMC - PubMed
-
- Britto, J. A. , Evans, R. D. , Hayward, R. D. , & Jones, B. M. (2001). From genotype to phenotype: the differential expression of FGF, FGFR, and TGFbeta genes characterizes human cranioskeletal development and reflects clinical presentation in FGFR syndromes. Plastic and Reconstructive Surgery, 108(7), 2026–2039. Discussion 2040‐2026 10.1097/00006534-200112000-00030 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

