Engineering a Rapid Insulin Release System Controlled By Oral Drug Administration
- PMID: 35048556
- PMCID: PMC8948567
- DOI: 10.1002/advs.202105619
Engineering a Rapid Insulin Release System Controlled By Oral Drug Administration
Abstract
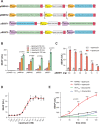
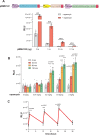
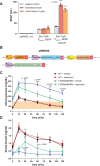
Rapid insulin release plays an essential role in maintaining blood-glucose homeostasis in mammalians. Patients diagnosed with type-I diabetes mellitus experience chronic and remarkably high blood-sugar levels, and require lifelong insulin injection therapy, so there is a need for more convenient and less invasive insulin delivery systems to increase patients' compliance and also to enhance their quality of life. Here, an endoplasmic-reticulum-localized split sec-tobacco etch virus protease (TEVp)-based rapamycin-actuated protein-induction device (RAPID) is engineered, which is composed of the rapamycin-inducible dimerization domains FK506 binding protein (FKBP) and FKBP-rapamycin binding protein fused with modified split sec-TEVp components. Insulin accumulation inside the endoplasmic reticulum (ER) is achieved through tagging its C-terminus with KDEL, an ER-retention signal, spaced by a TEVp cleavage site. In the presence of rapamycin, the split sec-TEVp-based RAPID components dimerize, regain their proteolytic activity, and remove the KDEL retention signal from insulin. This leads to rapid secretion of accumulated insulin from cells within few minutes. Using liver hydrodynamic transfection methodology, it is shown that RAPID quickly restores glucose homeostasis in type-1-diabetic (T1DM) mice treated with an oral dose of clinically licensed rapamycin. This rapid-release technology may become the foundation for other cell-based therapies requiring instantaneous biopharmaceutical availability.
Keywords: diabetes; endoplasmic reticulum; insulin; rapid release.
© 2022 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Fargion S., Dongiovanni P., Guzzo A., Colombo S., Valenti L., Fracanzani A. L., Aliment. Pharmacol. Ther. 2005, 22, 61. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
