Comparison of Different Dosages of Alteplase in Atrial Fibrillation-Related Acute Ischemic Stroke After Intravenous Thrombolysis: A Nationwide, Multicenter, Prospective Cohort Study in Taiwan
- PMID: 35048714
- PMCID: PMC9238492
- DOI: 10.1161/JAHA.121.023032
Comparison of Different Dosages of Alteplase in Atrial Fibrillation-Related Acute Ischemic Stroke After Intravenous Thrombolysis: A Nationwide, Multicenter, Prospective Cohort Study in Taiwan
Abstract
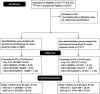
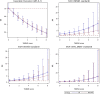
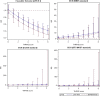
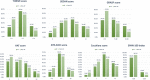
Background Insufficient evidence is available for patients with acute ischemic stroke with atrial fibrillation (AF) to determine the efficacy and safety of different dosages of intravenous thrombolysis treatment. This study examined clinical outcomes in Chinese patients with stroke with and without AF after intravenous thrombolysis treatment with different intravenous thrombolysis doses. Methods and Results This multicenter, prospective cohort study recruited 2351 patients with acute ischemic stroke (1371 with AF and 980 without AF) treated with intravenous thrombolysis using alteplase. The Totaled Health Risks in Vascular Events score is a validated risk-scoring tool used for assessing patients with acute ischemic stroke with and without AF. We evaluated favorable functional outcome at day 90 and symptomatic intracranial hemorrhage within 24 to 36 hours and outcomes of the patients receiving different doses of alteplase. Compared with the non-AF group, the AF group exhibited a 2- to 3-fold increased risk of symptomatic intracranial hemorrhage according to the National Institute of Neurological Disorders and Stroke standard (relative risk [RR], 2.10 [95% CI, 1.35-3.26]). Favorable functional outcome at 90 days and symptomatic intracranial hemorrhage rates according to the European Cooperative Acute Stroke Study II and the Safe Implementation of Thrombolysis in Stroke-Monitoring Study standards did not significantly differ between the AF and non-AF groups. In addition, the low-dose alteplase subgroup exhibited an increased risk of symptomatic intracranial hemorrhage according to the National Institute of Neurological Disorders and Stroke standard (RR, 2.84 [95% CI, 1.63-4.96]). A validation study confirmed these findings after adjustment for scores determined using different stroke risk-scoring tools. Conclusions Different alteplase dosages did not affect functional status at 90 days in the AF and non-AF groups. Thus, the adoption of low-dose alteplase simply because of AF is not recommended.
Keywords: acute stroke; atrial fibrillation; functional outcome; intravenous thrombolysis; symptomatic intracranial hemorrhage.
Figures


References
-
- Brachmann J, Morillo CA, Sanna T, Di Lazzaro V, Diener HC, Bernstein RA, Rymer M, Ziegler PD, Liu S, Passman RS. Uncovering atrial fibrillation beyond short‐term monitoring in cryptogenic stroke patients: three‐year results from the cryptogenic stroke and underlying atrial fibrillation trial. Circ Arrhythm Electrophysiol. 2016;9:e003333. doi: 10.1161/CIRCEP.115.003333 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

