VEGF signalling causes stalls in brain capillaries and reduces cerebral blood flow in Alzheimer's mice
- PMID: 35048960
- PMCID: PMC9150081
- DOI: 10.1093/brain/awab387
VEGF signalling causes stalls in brain capillaries and reduces cerebral blood flow in Alzheimer's mice
Abstract
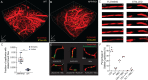
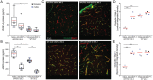
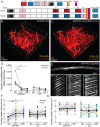
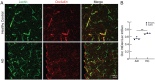
Increased incidence of stalled capillary blood flow caused by adhesion of leucocytes to the brain microvascular endothelium leads to a 17% reduction of cerebral blood flow and exacerbates short-term memory loss in multiple mouse models of Alzheimer's disease. Here, we report that vascular endothelial growth factor (VEGF) signalling at the luminal side of the brain microvasculature plays an integral role in the capillary stalling phenomenon of the APP/PS1 mouse model. Administration of the anti-mouse VEGF-A164 antibody, an isoform that inhibits blood-brain barrier hyperpermeability, reduced the number of stalled capillaries within an hour of injection, leading to an immediate increase in average capillary blood flow but not capillary diameter. VEGF-A inhibition also reduced the overall endothelial nitric oxide synthase protein concentrations, increased occludin levels and decreased the penetration of circulating Evans Blue dye across the blood-brain barrier into the brain parenchyma, suggesting increased blood-brain barrier integrity. Capillaries prone to neutrophil adhesion after anti-VEGF-A treatment also had lower occludin concentrations than flowing capillaries. Taken together, our findings demonstrate that VEGF-A signalling in APP/PS1 mice contributes to aberrant endothelial nitric oxide synthase /occludin-associated blood-brain barrier permeability, increases the incidence of capillary stalls, and leads to reductions in cerebral blood flow. Reducing leucocyte adhesion by inhibiting luminal VEGF signalling may provide a novel and well-tolerated strategy for improving brain microvascular blood flow in Alzheimer's disease patients.
Keywords: Alzheimer’s disease; blood–brain barrier; capillary stalling; cerebral blood flow; vascular endothelial growth factor.
© The Author(s) (2022). Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures



Similar articles
-
High-Density Lipoprotein Mimetic Peptide 4F Reduces Toxic Amyloid-Beta Exposure to the Blood-Brain Barrier Endothelium in Alzheimer's Disease Transgenic Mice.Mol Pharm. 2024 Nov 4;21(11):5661-5671. doi: 10.1021/acs.molpharmaceut.4c00633. Epub 2024 Oct 11. Mol Pharm. 2024. PMID: 39394037
-
Endothelial cell-selective adhesion molecule deficiency exhibits increased pulmonary vascular resistance due to impaired endothelial nitric oxide signaling.Am J Physiol Heart Circ Physiol. 2025 Feb 1;328(2):H283-H293. doi: 10.1152/ajpheart.00593.2024. Epub 2024 Dec 31. Am J Physiol Heart Circ Physiol. 2025. PMID: 39740345 Free PMC article.
-
Photobiomodulation mitigates blood-brain barrier disruption in APP/PS1 mouse model of Alzheimer's disease by activating the AMPK pathway.Alzheimers Res Ther. 2025 Jun 23;17(1):141. doi: 10.1186/s13195-025-01787-7. Alzheimers Res Ther. 2025. PMID: 40551167 Free PMC article.
-
Anti-vascular endothelial growth factor biosimilars for neovascular age-related macular degeneration.Cochrane Database Syst Rev. 2024 Jun 3;6(6):CD015804. doi: 10.1002/14651858.CD015804.pub2. Cochrane Database Syst Rev. 2024. PMID: 38829176 Free PMC article.
-
Anti-vascular endothelial growth factor for neovascular glaucoma.Cochrane Database Syst Rev. 2023 Apr 3;4(4):CD007920. doi: 10.1002/14651858.CD007920.pub4. Cochrane Database Syst Rev. 2023. PMID: 37010901 Free PMC article.
Cited by
-
Altered hemodynamics and vascular reactivity in a mouse model with severe pericyte deficiency.J Cereb Blood Flow Metab. 2023 May;43(5):763-777. doi: 10.1177/0271678X221147366. Epub 2022 Dec 22. J Cereb Blood Flow Metab. 2023. PMID: 36545806 Free PMC article.
-
Deep Imaging to Dissect Microvascular Contributions to White Matter Degeneration in Rodent Models of Dementia.Stroke. 2023 May;54(5):1403-1415. doi: 10.1161/STROKEAHA.122.037156. Epub 2023 Apr 24. Stroke. 2023. PMID: 37094035 Free PMC article. Review.
-
Pathophysiologic abnormalities in transgenic mice carrying the Alzheimer disease PSEN1 Δ440 mutation.Hum Mol Genet. 2024 Nov 20;33(23):2051-2070. doi: 10.1093/hmg/ddae139. Hum Mol Genet. 2024. PMID: 39323410
-
A pathogenic role for IL-10 signalling in capillary stalling and cognitive impairment in type 1 diabetes.Nat Metab. 2024 Nov;6(11):2082-2099. doi: 10.1038/s42255-024-01159-9. Epub 2024 Nov 4. Nat Metab. 2024. PMID: 39496927 Free PMC article.
-
Capillaries susceptible to frequent stall dynamics revealed by comparing OCT and bessel-2PM measurements.J Cereb Blood Flow Metab. 2025 Jun 25:271678X251353141. doi: 10.1177/0271678X251353141. Online ahead of print. J Cereb Blood Flow Metab. 2025. PMID: 40562716 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

