Xbp1 and Brachyury establish an evolutionarily conserved subcircuit of the notochord gene regulatory network
- PMID: 35049502
- PMCID: PMC8803312
- DOI: 10.7554/eLife.73992
Xbp1 and Brachyury establish an evolutionarily conserved subcircuit of the notochord gene regulatory network
Abstract
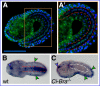
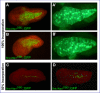
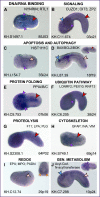
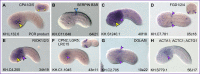
Gene regulatory networks coordinate the formation of organs and structures that compose the evolving body plans of different organisms. We are using a simple chordate model, the Ciona embryo, to investigate the essential gene regulatory network that orchestrates morphogenesis of the notochord, a structure necessary for the proper development of all chordate embryos. Although numerous transcription factors expressed in the notochord have been identified in different chordates, several of them remain to be positioned within a regulatory framework. Here, we focus on Xbp1, a transcription factor expressed during notochord formation in Ciona and other chordates. Through the identification of Xbp1-downstream notochord genes in Ciona, we found evidence of the early co-option of genes involved in the unfolded protein response to the notochord developmental program. We report the regulatory interplay between Xbp1 and Brachyury, and by extending these results to Xenopus, we show that Brachyury and Xbp1 form a cross-regulatory subcircuit of the notochord gene regulatory network that has been consolidated during chordate evolution.
Keywords: Brachyury; C. intestinalis; Ciona robusta; XBP1; Xenopus; developmental biology; evolutionary biology; gene regulatory network; notochord.
© 2022, Wu et al.
Conflict of interest statement
YW, AD, DJ, JK, LN, KB, JA, JS, AD No competing interests declared
Figures






References
-
- Baek HA, Kim DS, Park HS, Jang KY, Kang MJ, Lee DG, Moon WS, Chae HJ, Chung MJ. Involvement of endoplasmic reticulum stress in myofibroblastic differentiation of lung fibroblasts. American Journal of Respiratory Cell and Molecular Biology. 2012;46:731–739. doi: 10.1165/rcmb.2011-0121OC. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

