Integrity of the Intestinal Barrier: The Involvement of Epithelial Cells and Microbiota-A Mutual Relationship
- PMID: 35049768
- PMCID: PMC8772550
- DOI: 10.3390/ani12020145
Integrity of the Intestinal Barrier: The Involvement of Epithelial Cells and Microbiota-A Mutual Relationship
Abstract
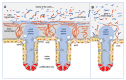
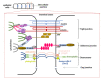
The gastrointestinal tract, which is constantly exposed to a multitude of stimuli, is considered responsible for maintaining the homeostasis of the host. It is inhabited by billions of microorganisms, the gut microbiota, which form a mutualistic relationship with the host. Although the microbiota is generally recognized as beneficial, at the same time, together with pathogens, they are a permanent threat to the host. Various populations of epithelial cells provide the first line of chemical and physical defense against external factors acting as the interface between luminal microorganisms and immunocompetent cells in lamina propria. In this review, we focus on some essential, innate mechanisms protecting mucosal integrity, thus responsible for maintaining intestine homeostasis. The characteristics of decisive cell populations involved in maintaining the barrier arrangement, based on mucus secretion, formation of intercellular junctions as well as production of antimicrobial peptides, responsible for shaping the gut microbiota, are presented. We emphasize the importance of cross-talk between gut microbiota and epithelial cells as a factor vital for the maintenance of the homeostasis of the GI tract. Finally, we discuss how the imbalance of these regulations leads to the compromised barrier integrity and dysbiosis considered to contribute to inflammatory disorders and metabolic diseases.
Keywords: Paneth cells; epithelial cells; gastrointestinal tract; goblet cells; microbiota.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

