Bacillimidazoles A-F, Imidazolium-Containing Compounds Isolated from a Marine Bacillus
- PMID: 35049898
- PMCID: PMC8779896
- DOI: 10.3390/md20010043
Bacillimidazoles A-F, Imidazolium-Containing Compounds Isolated from a Marine Bacillus
Abstract
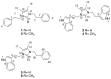
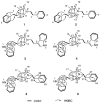
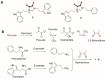
Chemical investigations of a marine sponge-associated Bacillus revealed six new imidazolium-containing compounds, bacillimidazoles A-F (1-6). Previous reports of related imidazolium-containing natural products are rare. Initially unveiled by timsTOF (trapped ion mobility spectrometry) MS data, extensive HRMS and 1D and 2D NMR analyses enabled the structural elucidation of 1-6. In addition, a plausible biosynthetic pathway to bacillimidazoles is proposed based on isotopic labeling experiments and invokes the highly reactive glycolytic adduct 2,3-butanedione. Combined, the results of structure elucidation efforts, isotopic labeling studies and bioinformatics suggest that 1-6 result from a fascinating intersection of primary and secondary metabolic pathways in Bacillus sp. WMMC1349. Antimicrobial assays revealed that, of 1-6, only compound six displayed discernible antibacterial activity, despite the close structural similarities shared by all six natural products.
Keywords: antibacterial; biosynthetic gene cluster; heterocycles; imidazolium; isotopic enrichment; marine-derived Bacillus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
MeSH terms
Substances
Grants and funding
- multiple funds through School of Pharmacy & the Graduate School/University of Wisconsin-Madison
- OIA-9977484/National Science Foundation
- unknown/United States Department of Agriculture
- U19 AI109673/AI/NIAID NIH HHS/United States
- RR02781/National Institute of Health
- BIR-9214394/National Science Foundation
- U19AI141720/NH/NIH HHS/United States
- U19AI109673/National Institute of Health
- Postdoctoral Mobility Fellowship/SNSF_/Swiss National Science Foundation/Switzerland
- P41GM103399/National Institute of Health
- U19 AI142720/AI/NIAID NIH HHS/United States
- R01AT009874/National Institute of Health
- RR08438/National Institute of Health
- DMB-8415048/National Science Foundation
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

