Automated Sample Preparation and Data Collection Workflow for High-Throughput In Vitro Metabolomics
- PMID: 35050173
- PMCID: PMC8778710
- DOI: 10.3390/metabo12010052
Automated Sample Preparation and Data Collection Workflow for High-Throughput In Vitro Metabolomics
Abstract
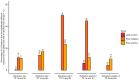
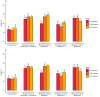
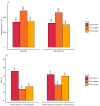
Regulatory bodies have started to recognise the value of in vitro screening and metabolomics as two types of new approach methodologies (NAMs) for chemical risk assessments, yet few high-throughput in vitro toxicometabolomics studies have been reported. A significant challenge is to implement automated sample preparation of the low biomass samples typically used for in vitro screening. Building on previous work, we have developed, characterised and demonstrated an automated sample preparation and analysis workflow for in vitro metabolomics of HepaRG cells in 96-well microplates using a Biomek i7 Hybrid Workstation (Beckman Coulter) and Orbitrap Elite (Thermo Scientific) high-resolution nanoelectrospray direct infusion mass spectrometry (nESI-DIMS), across polar metabolites and lipids. The experimental conditions evaluated included the day of metabolite extraction, order of extraction of samples in 96-well microplates, position of the 96-well microplate on the instrument's deck and well location within a microplate. By using the median relative standard deviation (mRSD (%)) of spectral features, we have demonstrated good repeatability of the workflow (final mRSD < 30%) with a low percentage of features outside the threshold applied for statistical analysis. To improve the quality of the automated workflow further, small method modifications were made and then applied to a large cohort study (4860 sample infusions across three nESI-DIMS assays), which confirmed very high repeatability of the whole workflow from cell culturing to metabolite measurements, whilst providing a significant improvement in sample throughput. It is envisioned that the automated in vitro metabolomics workflow will help to advance the application of metabolomics (as a part of NAMs) in chemical safety, primarily as an approach for high throughput screening and prioritisation.
Keywords: automation; direct infusion mass spectrometry; high-throughput screening; in vitro metabolomics; sample preparation.
Conflict of interest statement
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
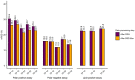

References
-
- Becker R.A. Transforming regulatory safety evaluations using New Approach Methodologies: A perspective of an industrial toxicologist. Curr. Opin. Toxicol. 2019;15:93–98. doi: 10.1016/j.cotox.2019.07.002. - DOI
-
- Pistollato F., Madia F., Corvi R., Munn S., Grignard E., Paini A., Worth A., Bal-Price A., Prieto P., Casati S., et al. Current EU Regulatory Requirements for the Assessment of Chemicals and Cosmetic Products: Challenges and Opportunities for introducing New Approach Methodologies. Springer; Berlin/Heidelberg, Germany: 2021. - PMC - PubMed
-
- Viant M.R., Ebbels T.M.D., Beger R.D., Ekman D.R., Epps D.J.T., Kamp H., Leonards P.E.G., Loizou G.D., MacRae J.I., van Ravenzwaay B., et al. Use cases, best practice and reporting standards for metabolomics in regulatory toxicology. Nat. Commun. 2019;10:3041. doi: 10.1038/s41467-019-10900-y. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

