Characterizing Neonatal Heart Maturation, Regeneration, and Scar Resolution Using Spatial Transcriptomics
- PMID: 35050211
- PMCID: PMC8779463
- DOI: 10.3390/jcdd9010001
Characterizing Neonatal Heart Maturation, Regeneration, and Scar Resolution Using Spatial Transcriptomics
Abstract
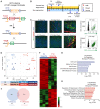
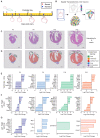
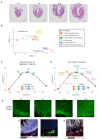
The neonatal mammalian heart exhibits a remarkable regenerative potential, which includes fibrotic scar resolution and the generation of new cardiomyocytes. To investigate the mechanisms facilitating heart repair after apical resection in neonatal mice, we conducted bulk and spatial transcriptomic analyses at regenerative and non-regenerative timepoints. Importantly, spatial transcriptomics provided near single-cell resolution, revealing distinct domains of atrial and ventricular myocardium that exhibit dynamic phenotypic alterations during postnatal heart maturation. Spatial transcriptomics also defined the cardiac scar, which transitions from a proliferative to secretory phenotype as the heart loses regenerative potential. The resolving scar is characterized by spatially and temporally restricted programs of inflammation, epicardium expansion and extracellular matrix production, metabolic reprogramming, lipogenic scar extrusion, and cardiomyocyte restoration. Finally, this study revealed the emergence of a regenerative border zone defined by immature cardiomyocyte markers and the robust expression of Sprr1a. Taken together, our study defines the spatially and temporally restricted gene programs that underlie neonatal heart regeneration and provides insight into cardio-restorative mechanisms supporting scar resolution.
Keywords: fibroblast; heart; mouse; regeneration; scar; spatial transcriptomics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Virani S.S., Alonso A., Aparicio H.J., Benjamin E.J., Bittencourt M.S., Callaway C.W., Carson A.P., Chamberlain A.M., Cheng S., Delling F.N., et al. Heart Disease and Stroke Statistics-2021 Update: A Report From the American Heart Association. Circulation. 2021;143:e254–e743. doi: 10.1161/CIR.0000000000000950. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

