Impact of the COVID-19 Pandemic on Non-COVID-19 Clinical Trials
- PMID: 35050229
- PMCID: PMC8781416
- DOI: 10.3390/jcdd9010019
Impact of the COVID-19 Pandemic on Non-COVID-19 Clinical Trials
Abstract
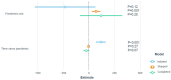
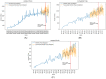
Randomized controlled trials (RCT) were impacted by the COVID-19 pandemic, but no systematic analysis has evaluated the overall impact of COVID-19 on non-COVID-19-related RCTs. The ClinicalTrials.gov database was queried in February 2020. Eligible studies included all randomized trials with a start date after 1 January 2010 and were active during the period from 1 January 2015 to 31 December 2020. The effect of the pandemic period on non-COVID-19 trials was determined by piece-wise regression models using 11 March 2020 as the start of the pandemic and by time series analysis (models fitted using 2015-2018 data and forecasted for 2019-2020). The study endpoints were early trial stoppage, normal trial completion, and trial activation. There were 161,377 non-COVID-19 trials analyzed. The number of active trials increased annually through 2019 but decreased in 2020. According to the piece-wise regression models, trial completion was not affected by the pandemic (p = 0.56) whereas trial stoppage increased (p = 0.001). There was a pronounced decrease in trial activation early during the pandemic (p < 0.001) which then recovered. The findings from the time series models were consistent comparing forecasted and observed results (trial completion p = 0.22; trial stoppage p < 0.01; trial activation, p = 0.01). During the pandemic, there was an increase in non-COVID-19 RCTs stoppage without changes in RCT completion. There was a sharp decline in new RCTs at the beginning of the pandemic, which later recovered.
Keywords: COVID-19; ClinicalTrials.gov; randomized controlled trials.
Conflict of interest statement
Deepak L. Bhatt discloses the following relationships—Advisory Board: Cardax, CellProthera, Cereno Scientific, Elsevier Practice Update Cardiology, Janssen, Level Ex, Medscape Cardiology, MyoKardia, Novo Nordisk, PhaseBio, PLx Pharma, Regado Biosciences; Board of Directors: Boston VA Research Institute, Society of Cardiovascular Patient Care, TobeSoft; Chair: American Heart Association Quality Oversight Committee; Data Monitoring Committees: Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Cleveland Clinic (including for the ExCEED trial, funded by Edwards), Contego Medical (Chair, PERFORMANCE 2), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo), Population Health Research Institute; Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Vice-Chair, ACC Accreditation Committee), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE-DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim; AEGIS-II executive committee funded by CSL Behring), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Canadian Medical and Surgical Knowledge Translation Research Group (clinical trial steering committees), Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), K2P (Co-Chair, interdisciplinary curriculum), Level Ex, Medtelligence/ReachMD (CME steering committees), MJH Life Sciences, Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and USA national co-leader, funded by Bayer), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees); Other: Clinical Cardiology (Deputy Editor), NCDR-ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); Research Funding: Abbott, Afimmune, Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Cardax, Chiesi, CSL Behring, Eisai, Ethicon, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, HLS Therapeutics, Idorsia, Ironwood, Ischemix, Janssen, Lexicon, Lilly, Medtronic, MyoKardia, Novo Nordisk, Owkin, Pfizer, PhaseBio, PLx Pharma, Regeneron, Roche, Sanofi, Synaptic, The Medicines Company; Royalties: Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald’s Heart Disease); Site Co-Investigator: Abbott, Biotronik, Boston Scientific, CSI, St. Jude Medical (now Abbott), Svelte; Trustee: American College of Cardiology; Unfunded Research: FlowCo, Merck, Takeda. The remaining authors declare no conflict of interest.
Figures

References
-
- Johns Hopkins University Coronavirus Resource Center. [(accessed on 8 January 2021)]. Available online: https://coronavirus.jhu.edu/
-
- Fanaroff A.C., Califf R.M., Harrington R.A., Granger C.B., McMurray J.J.V., Patel M.R., Bhatt D.L., Windecker S., Hernandez A.F., Gibson C.M., et al. Randomized Trials Versus Common Sense and Clinical Observation. J. Am. Coll. Cardiol. 2020;76:580–589. doi: 10.1016/j.jacc.2020.05.069. - DOI - PMC - PubMed
-
- Varshney A.S., Wang D.E., Bhatt A.S., Blood A., Sharkawi M.A., Siddiqi H.K., Vaduganathan M., Monteleone P.P., Patel M.R., Jones W.S., et al. Characteristics of clinical trials evaluating cardiovascular therapies for Coronavirus Disease 2019 Registered on ClinicalTrials.gov: A cross sectional analysis. Am. Heart J. 2021;232:105–115. doi: 10.1016/j.ahj.2020.10.065. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

