Interplay Between Duration of Androgen Deprivation Therapy and External Beam Radiotherapy With or Without a Brachytherapy Boost for Optimal Treatment of High-risk Prostate Cancer: A Patient-Level Data Analysis of 3 Cohorts
- PMID: 35050303
- PMCID: PMC8778608
- DOI: 10.1001/jamaoncol.2021.6871
Interplay Between Duration of Androgen Deprivation Therapy and External Beam Radiotherapy With or Without a Brachytherapy Boost for Optimal Treatment of High-risk Prostate Cancer: A Patient-Level Data Analysis of 3 Cohorts
Abstract
Importance: Radiotherapy combined with androgen deprivation therapy (ADT) is a standard of care for high-risk prostate cancer. However, the interplay between radiotherapy dose and the required minimum duration of ADT is uncertain.
Objective: To determine the specific ADT duration threshold that provides a distant metastasis-free survival (DMFS) benefit in patients with high-risk prostate cancer receiving external beam radiotherapy (EBRT) or EBRT with a brachytherapy boost (EBRT+BT).
Design, settings, and participants: This was a cohort study of 3 cohorts assembled from a multicenter retrospective study (2000-2013); a post hoc analysis of the Randomized Androgen Deprivation and Radiotherapy 03/04 (RADAR; 2003-2007) randomized clinical trial (RCT); and a cross-trial comparison of the RADAR vs the Deprivación Androgénica y Radio Terapía (Androgen Deprivation and Radiation Therapy; DART) 01/05 RCT (2005-2010). In all, the study analyzed 1827 patients treated with EBRT and 1108 patients treated with EBRT+BT from the retrospective cohort; 181 treated with EBRT and 203 with EBRT+BT from RADAR; and 91 patients treated with EBRT from DART. The study was conducted from October 15, 2020, to July 1, 2021, and the data analyses, from January 5 to June 15, 2021.
Exposures: High-dose EBRT or EBRT+BT for an ADT duration determined by patient-physician choice (retrospective) or by randomization (RCTs).
Main outcomes and measures: The primary outcome was DMFS; secondary outcome was overall survival (OS). Natural cubic spline analysis identified minimum thresholds (months).
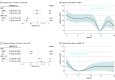
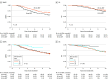
Results: This cohort study of 3 studies totaling 3410 men (mean age [SD], 68 [62-74] years; race and ethnicity not collected) with high-risk prostate cancer found a significant interaction between the treatment type (EBRT vs EBRT+BT) and ADT duration (binned to <6, 6 to <18, and ≥18 months). Natural cubic spline analysis identified minimum duration thresholds of 26.3 months (95% CI, 25.4-36.0 months) for EBRT and 12 months (95% CI, 4.9-36.0 months) for EBRT+BT for optimal effect on DMFS. In RADAR, the prolongation of ADT for patients receiving only EBRT was not associated with significant improvements in DMFS (hazard ratio [HR], 1.01; 95% CI, 0.65-1.57); however, for patients receiving EBRT+BT, a longer duration was associated with improved DMFS (DMFS HR, 0.56; 95% CI, 0.36-0.87; P = .01). For patients receiving EBRT alone (DART), 28 months of ADT was associated with improved DMFS compared with 18 months (RADAR HR, 0.37; 95% CI, 0.17-0.80; P = .01).
Conclusions and relevance: These cohort study findings suggest that the optimal minimum ADT duration for treatment with high-dose EBRT alone is more than 18 months; and for EBRT+BT, it is 18 months or possibly less. Additional studies are needed to determine more precise minimum durations.
Conflict of interest statement
Figures
Comment in
-
Urological Oncology: Prostate Cancer.J Urol. 2023 Mar;209(3):635-637. doi: 10.1097/JU.0000000000003088. Epub 2022 Dec 1. J Urol. 2023. PMID: 36453267 No abstract available.
References
-
- Denham JW, Joseph D, Lamb DS, et al. . Short-term androgen suppression and radiotherapy versus intermediate-term androgen suppression and radiotherapy, with or without zoledronic acid, in men with locally advanced prostate cancer (TROG 03.04 RADAR): 10-year results from a randomised, phase 3, factorial trial. Lancet Oncol. 2019;20(2):267-281. doi:10.1016/S1470-2045(18)30757-5 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

