Autofluorescent Organelles Within the Retinal Pigment Epithelium in Human Donor Eyes With and Without Age-Related Macular Degeneration
- PMID: 35050307
- PMCID: PMC8787573
- DOI: 10.1167/iovs.63.1.23
Autofluorescent Organelles Within the Retinal Pigment Epithelium in Human Donor Eyes With and Without Age-Related Macular Degeneration
Abstract
Purpose: Human retinal pigment epithelium (RPE) cells contain lipofuscin, melanolipofuscin, and melanosome organelles that impact clinical autofluorescence (AF) imaging. Here, we quantified the effect of age-related macular degeneration (AMD) on granule count and histologic AF of RPE cell bodies.
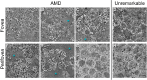
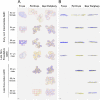
Methods: Seven AMD-affected human RPE-Bruch's membrane flatmounts (early and intermediate = 3, late dry = 1, and neovascular = 3) were imaged at fovea, perifovea, and near periphery using structured illumination and confocal AF microscopy (excitation 488 nm) and compared to RPE-flatmounts with unremarkable macula (n = 7, >80 years). Subsequently, granules were marked with computer assistance, and classified by their AF properties. The AF/cell was calculated from confocal images. The total number of granules and AF/cell was analyzed implementing a mixed effect analysis of covariance (ANCOVA).
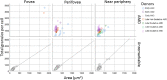
Results: A total of 152 AMD-affected RPE cells were analyzed (fovea = 22, perifovea = 60, and near-periphery = 70). AMD-affected RPE cells showed increased variability in size and a significantly increased granule load independent of the retinal location (fovea: P = 0.02, perifovea: P = 0.04, and near periphery: P < 0.01). The lipofuscin fraction of total organelles decreased and the melanolipofuscin fraction increased in AMD, at all locations (especially the fovea). AF was significantly lower in AMD-affected cells (fovea: <0.01, perifovea: <0.01, and near periphery: 0.02).
Conclusions: In AMD RPE, lipofuscin was proportionately lowest in the fovea, a location also known to be affected by accumulation of soft drusen and preservation of cone-mediated visual acuity. Enlarged RPE cell bodies displayed increased net granule count but diminished total AF. Future studies should also assess the impact on AF imaging of RPE apical processes containing melanosomes.
Conflict of interest statement
Disclosure:
Figures
References
-
- Strauss O. The retinal pigment epithelium in visual function. Physiol Rev. 2005; 85(3): 845–881. - PubMed
-
- Pollreisz A, Messinger JD, Sloan KR, et al. .. Visualizing melanosomes, lipofuscin, and melanolipofuscin in human retinal pigment epithelium using serial block face scanning electron microscopy. Exp Eye Res. 2018; 166: 131–139. - PubMed
-
- Feeney-Burns L, Hilderbrand ES, Eldridge S.. Aging human RPE: morphometric analysis of macular, equatorial, and peripheral cells. Invest Ophthalmol Vis Sci. 1984; 25(2): 195–200. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

