Acute Myeloid Leukemia Stem Cells: Origin, Characteristics, and Clinical Implications
- PMID: 35050458
- PMCID: PMC10942736
- DOI: 10.1007/s12015-021-10308-6
Acute Myeloid Leukemia Stem Cells: Origin, Characteristics, and Clinical Implications
Abstract
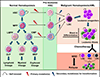
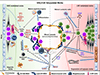
The stem cells of acute myeloid leukemia (AML) are the malignancy initiating cells whose survival ultimately drives growth of these lethal diseases. Here we review leukemia stem cell (LSC) biology, particularly as it relates to the very heterogeneous nature of AML and to its high disease relapse rate. Leukemia ontogeny is presented, and the defining functional and phenotypic features of LSCs are explored. Surface and metabolic phenotypes of these cells are described, particularly those that allow distinction from features of normal hematopoietic stem cells (HSCs). Opportunities for use of this information for improving therapy for this challenging group of diseases is highlighted, and we explore the clinical needs which may be addressed by emerging LSC data. Finally, we discuss current gaps in the scientific understanding of LSCs.
Keywords: Acute Myeloid Leukemia; Hematopoiesis; Leukemia; Leukemia Stem Cell.
© 2021. The Author(s), under exclusive licence to Springer Science+Business Media, LLC, part of Springer Nature.
Conflict of interest statement
Conflicts of Interest None Relevant
Figures
Comment in
-
Paper of April Issue of Stem Cell Reviews and Reports Presents an Update on Acute Myeloid Leukemia Stem Cells: Origin, Characteristics, and Clinical Implications by Dr. David F. Claxton et al.Stem Cell Rev Rep. 2022 Apr;18(4):1209-1210. doi: 10.1007/s12015-022-10365-5. Stem Cell Rev Rep. 2022. PMID: 35286595 No abstract available.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

