ERBB2 Copy Number as a Quantitative Biomarker for Real-World Outcomes to Anti-Human Epidermal Growth Factor Receptor 2 Therapy in Advanced Gastroesophageal Adenocarcinoma
- PMID: 35050711
- PMCID: PMC8789214
- DOI: 10.1200/PO.21.00330
ERBB2 Copy Number as a Quantitative Biomarker for Real-World Outcomes to Anti-Human Epidermal Growth Factor Receptor 2 Therapy in Advanced Gastroesophageal Adenocarcinoma
Abstract
Purpose: Human epidermal growth factor receptor 2 (HER2) overexpression or amplification (ERBB2amp) are biomarkers for approved anti-HER2 therapies. ERBB2amp may better predict response compared with immunohistochemistry or in situ hybridization, and quantitative copy number (CN) may further stratify patients. We characterized ERBB2amp in advanced gastroesophageal adenocarcinomas (GEA) and hypothesized that increased CN was associated with better outcome to trastuzumab.
Methods: Comprehensive genomic profiling, including assessment of ERBB2amp, was performed for 12,905 GEA tissue cases. Clinical outcomes were assessed using a clinicogenomic database linking deidentified electronic health record-derived clinical data to genomic data. Multivariable Cox proportional hazard models were used for real-world progression-free survival (rwPFS) comparisons.
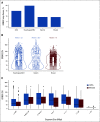
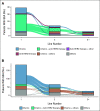
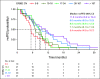
Results: ERBB2amp (CN ≥ 5) was detected in 15% (1,934 of 12,905) of GEA; median CN 22 (interquartile range 9-73). Median ERBB2 amplicon size was 0.27 megabase (interquartile range 0.13-0.95), and smaller amplicons were associated with higher CN (P < .001). In the clinicogenomic database, of 101 evaluable first-line trastuzumab-treated patients, ERBB2 CN was a significant predictor of rwPFS as a continuous variable (adjusted hazard ratio = 0.73; 95% CI, 0.60 to 0.89; P = .002), whereas ERBB2 CN was not predictive of rwPFS on chemotherapy (adjusted hazard ratio = 0.93; 95% CI, 0.73 to 1.20; P = .59). Among trastuzumab-treated patients, no significant associations with ERBB2 CN were observed for disease site, age, stage at advanced diagnosis, or most selected coalterations.
Conclusion: ERBB2amp was detected in 15% of GEA tissue samples, with significant diversity in ERBB2 CN and amplicon focality. ERBB2 CN was predictive of rwPFS as a continuous variable for patients treated with trastuzumab. Further studies exploring the clinical utility of quantitative ERBB2 CN, particularly in the setting of the evolving anti-HER2 landscape and combination therapies, are warranted.
Conflict of interest statement
Figures
Similar articles
-
Association of ERBB2 Copy Number and Gene Coalterations With Trastuzumab Efficacy and Resistance in Human Epidermal Growth Factor Receptor 2-Positive Esophagogastric and Gastric Cancer.JCO Precis Oncol. 2022 Aug;6:e2200135. doi: 10.1200/PO.22.00135. JCO Precis Oncol. 2022. PMID: 35952320 Free PMC article.
-
Real-world association of HER2/ERBB2 concordance with trastuzumab clinical benefit in advanced esophagogastric cancer.Future Oncol. 2021 Nov;17(31):4101-4114. doi: 10.2217/fon-2021-0203. Epub 2021 Aug 31. Future Oncol. 2021. PMID: 34463133
-
FGFR2-Altered Gastroesophageal Adenocarcinomas Are an Uncommon Clinicopathologic Entity with a Distinct Genomic Landscape.Oncologist. 2019 Nov;24(11):1462-1468. doi: 10.1634/theoncologist.2019-0121. Epub 2019 Jun 27. Oncologist. 2019. PMID: 31249137 Free PMC article.
-
Progress and challenges in HER2-positive gastroesophageal adenocarcinoma.J Hematol Oncol. 2019 May 17;12(1):50. doi: 10.1186/s13045-019-0737-2. J Hematol Oncol. 2019. PMID: 31101074 Free PMC article. Review.
-
HER2 testing of gastro-oesophageal adenocarcinoma: a commentary and guidance document from the Association of Clinical Pathologists Molecular Pathology and Diagnostics Committee.J Clin Pathol. 2018 May;71(5):388-394. doi: 10.1136/jclinpath-2017-204943. Epub 2018 Feb 8. J Clin Pathol. 2018. PMID: 29439009 Review.
Cited by
-
Clinical application of targeted tumour sequencing tests for detecting ERBB2 amplification and optimizing anti-HER2 therapy in gastric cancer.BMC Cancer. 2024 Jun 11;24(1):719. doi: 10.1186/s12885-024-12482-5. BMC Cancer. 2024. PMID: 38862927 Free PMC article.
-
HER2 Copy Number and Resistance Mechanisms in Patients with HER2-positive Advanced Gastric Cancer Receiving Initial Trastuzumab-based Therapy in JACOB Trial.Clin Cancer Res. 2023 Feb 1;29(3):571-580. doi: 10.1158/1078-0432.CCR-22-2533. Clin Cancer Res. 2023. PMID: 36413222 Free PMC article.
-
Targeting HER2 in Gastroesophageal Cancer: A New Appetite for an Old Plight.Drugs. 2025 Mar;85(3):361-383. doi: 10.1007/s40265-024-02132-2. Epub 2025 Jan 23. Drugs. 2025. PMID: 39843758 Review.
-
Putative Clinical Potential of ERBB2 Amplification Assessment by ddPCR in FFPE-DNA and cfDNA of Gastroesophageal Adenocarcinoma Patients.Cancers (Basel). 2022 Apr 27;14(9):2180. doi: 10.3390/cancers14092180. Cancers (Basel). 2022. PMID: 35565309 Free PMC article.
-
Clinical outcomes and ctDNA correlates for CAPOX BETR: a phase II trial of capecitabine, oxaliplatin, bevacizumab, trastuzumab in previously untreated advanced HER2+ gastroesophageal adenocarcinoma.Nat Commun. 2024 Aug 9;15(1):6833. doi: 10.1038/s41467-024-51271-3. Nat Commun. 2024. PMID: 39122726 Free PMC article. Clinical Trial.
References
-
- Pegram MD, Lipton A, Hayes DF, et al. Phase II study of receptor-enhanced chemosensitivity using recombinant humanized anti-p185HER2/neu monoclonal antibody plus cisplatin in patients with HER2/neu-overexpressing metastatic breast cancer refractory to chemotherapy treatment J Clin Oncol 162659–26711998 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

