Human interictal epileptiform discharges are bidirectional traveling waves echoing ictal discharges
- PMID: 35050851
- PMCID: PMC8813051
- DOI: 10.7554/eLife.73541
Human interictal epileptiform discharges are bidirectional traveling waves echoing ictal discharges
Abstract
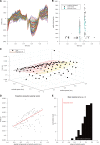
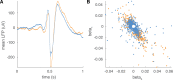
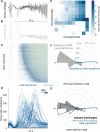
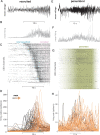
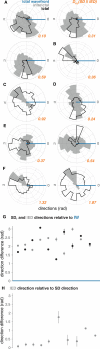
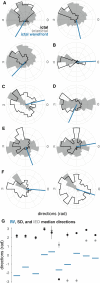
Interictal epileptiform discharges (IEDs), also known as interictal spikes, are large intermittent electrophysiological events observed between seizures in patients with epilepsy. Although they occur far more often than seizures, IEDs are less studied, and their relationship to seizures remains unclear. To better understand this relationship, we examined multi-day recordings of microelectrode arrays implanted in human epilepsy patients, allowing us to precisely observe the spatiotemporal propagation of IEDs, spontaneous seizures, and how they relate. These recordings showed that the majority of IEDs are traveling waves, traversing the same path as ictal discharges during seizures, and with a fixed direction relative to seizure propagation. Moreover, the majority of IEDs, like ictal discharges, were bidirectional, with one predominant and a second, less frequent antipodal direction. These results reveal a fundamental spatiotemporal similarity between IEDs and ictal discharges. These results also imply that most IEDs arise in brain tissue outside the site of seizure onset and propagate toward it, indicating that the propagation of IEDs provides useful information for localizing the seizure focus.
Keywords: computational biology; epilepsy; field potentials; human; interictal epileptiform discharges; neurons; neuroscience; seizures; systems biology.
© 2022, Smith et al.
Conflict of interest statement
ES, JL, EM, TD, KT, BG, RE, RG, CS No competing interests declared, PH is affiliated with Neurosurgical Associates, LLC. The author has no financial interests to declare, GM reports fees from Koh Young, Inc, SS consulting for Boston Scientific, Abbott, Neuropace, Zimmer Biomet, JR reports fees from Medtronic, Inc
Figures







Comment in
-
Interictal Discharges: All Roads Lead to Rome?Epilepsy Curr. 2022 Jul 16;22(4):252-254. doi: 10.1177/15357597221098809. eCollection 2022 Jul-Aug. Epilepsy Curr. 2022. PMID: 36187148 Free PMC article. No abstract available.
References
-
- Alarcon G, Garcia Seoane JJ, Binnie CD, Martin Miguel MC, Juler J, Polkey CE, Elwes RD, Ortiz Blasco JM. Origin and propagation of interictal discharges in the acute electrocorticogram. Implications for pathophysiology and surgical treatment of temporal lobe epilepsy. Brain. 1997;120 (Pt 12):2259–2282. doi: 10.1093/brain/120.12.2259. - DOI - PubMed
-
- Berens P. CircStat: A MATLAB Toolbox for Circular Statistics. J Stat Soft. 2009;31:v031. doi: 10.18637/jss.v031.i10. - DOI
-
- Berens P, Sinz F, Haddad A, Krause M, Zittrell F, Zavatone-Veth J. circstat-matlab. 302acc1GitHub. 2019 https://github.com/circstat/circstat-matlab

