Diagnostic accuracy of three commercially available one step RT-PCR assays for the detection of SARS-CoV-2 in resource limited settings
- PMID: 35051204
- PMCID: PMC8775315
- DOI: 10.1371/journal.pone.0262178
Diagnostic accuracy of three commercially available one step RT-PCR assays for the detection of SARS-CoV-2 in resource limited settings
Abstract
Background: COVID-19 is an ongoing public health pandemic regardless of the countless efforts made by various actors. Quality diagnostic tests are important for early detection and control. Notably, several commercially available one step RT-PCR based assays have been recommended by the WHO. Yet, their analytic and diagnostic performances have not been well documented in resource-limited settings. Hence, this study aimed to evaluate the diagnostic sensitivities and specificities of three commercially available one step reverse transcriptase-polymerase chain reaction (RT-PCR) assays in Ethiopia in clinical setting.
Methods: A cross-sectional study was conducted from April to June, 2021 on 279 respiratory swabs originating from community surveillance, contact cases and suspect cases. RNA was extracted using manual extraction method. Master-mix preparation, amplification and result interpretation was done as per the respective manufacturer. Agreements between RT-PCRs were analyzed using kappa values. Bayesian latent class models (BLCM) were fitted to obtain reliable estimates of diagnostic sensitivities, specificities of the three assays and prevalence in the absence of a true gold standard.
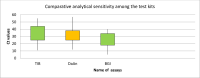
Results: Among the 279 respiratory samples, 50(18%), 59(21.2%), and 69(24.7%) were tested positive by TIB, Da An, and BGI assays, respectively. Moderate to substantial level of agreement was reported among the three assays with kappa value between 0 .55 and 0.72. Based on the BLCM relatively high specificities (95% CI) of 0.991(0.973-1.000), 0.961(0.930-0.991) and 0.916(0.875-0.952) and considerably lower sensitivities with 0.813(0.658-0.938), 0.836(0.712-0.940) and 0.810(0.687-0.920) for TIB MOLBIOL, Da An and BGI respectively were found.
Conclusions: While all the three RT-PCR assays displayed comparable sensitivities, the specificities of TIB MOLBIOL and Da An were considerably higher than BGI. These results help adjust the apparent prevalence determined by the three RT-PCRs and thus support public health decisions in resource limited settings and consider alternatives as per their prioritization matrix.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- University JH. Johns Hopkins University, J.H., worldometers COVID 19 Daily update. worldometers. Available from: https://coronavirus.jhu.edu/. Accessed October 13, 2021.
-
- World health organization. Coronavirus disease (COVID-19) Weekly Epidemiological Update and Weekly Operational Update. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situatio.... Accessed 13 October,2021.
-
- Luigi C., Fulvio P., Caterina L. et al.,. COVID-19 Pandemic: Prevention and Protection Measures to Be Adopted at the Workplace. Sustainability 2020, 12, 3603.; doi: 10.3390/su12093603 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous