The dawn of a new era of targeted lipid-lowering therapies
- PMID: 35051271
- PMCID: PMC9448630
- DOI: 10.1093/eurheartj/ehab841
The dawn of a new era of targeted lipid-lowering therapies
Abstract
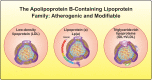
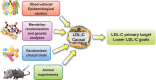
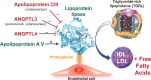
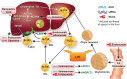
Lipid risk factors for cardiovascular disease depend in part on lifestyle, but optimum control of lipids often demands additional measures. Low-density lipoprotein (LDL) doubtless contributes causally to atherosclerosis. Recent human genetic findings have substantiated a number of novel targets for lipid-lowering therapy including apolipoprotein C-III, angiopoietin-like protein 3 and 4, apolipoprotein V, and ATP citrate lyase. These discoveries coupled with advances in biotechnology development afford new avenues for management of LDL and other aspects of lipid risk. Beyond LDL, new treatments targeting triglyceride-rich lipoproteins and lipoprotein(a) have become available and have entered clinical development. Biological and RNA-directed agents have joined traditional small-molecule approaches, which themselves have undergone considerable refinement. Innovative targeting strategies have increased efficacy of some of these novel interventions and markedly improved their tolerability. Gene-editing approaches have appeared on the horizon of lipid management. This article reviews this progress offering insight into novel biological and therapeutic discoveries, and places them into a practical patient care perspective.
Keywords: Angiopoietin-like proteins; Apolipoprotein C-III; Lipoprotein(a); Lipoproteins; RNA therapeutics; Triglycerides.
Published on behalf of the European Society of Cardiology. All rights reserved. © The Author(s) 2022. For permissions, please email: journals.permissions@oup.com.
Conflict of interest statement
Conflict of interest: L.T. reports consulting fees from Abbott, Amgen, Bayer, MSD, Mylan, Novartis, Sanofi, and Daiichi Sankyo; payment or honoraria from Abbott, Janssen, Amgen, Bayer, Daiichi Sankyo, MSD, Mylan, Novartis, Novo Nordisk, Sanofi, Servier, Pfizer, and Recordati Abdi-İbrahim; support for attending meetings for Servier; and participation on a data safety monitoring board or advisory board for Abbott, Amgen, Novartis, Sanofi, Daiichi-Sankyo, Mylan, and Pfizer. L.T. was the Past President European Atherosclerosis Society and the Past President Turkish Society of Cardiology. P.L. is an unpaid consultant to, or involved in clinical trials for Amgen, AstraZeneca, Baim Institute, Beren Therapeutics, Caristo, Esperion Therapeutics, Genentech, Kancera, Kowa Pharmaceuticals, Medimmune, Merck, Novo Nordisk, Merck, Novartis, Pfizer, and Sanofi–Regeneron. P.L is a member of scientific advisory board for Amgen, Corvidia Therapeutics, DalCor Pharmaceuticals, Kowa Pharmaceuticals, Olatec Therapeutics, Medimmune, Novartis, and XBiotech, Inc. P.L.’s laboratory has received research funding in the last 2 years from Novartis. P.L. is on the Board of Directors of XBiotech, Inc. and has a financial interest in Xbiotech, a company developing therapeutic human antibodies. P.L.’s interests were reviewed and are managed by Brigham and Women’s Hospital and Partners HealthCare in accordance with their conflict-of-interest policies.
Figures



References
-
- Libby P, Buring JE, Badimon Let al. Atherosclerosis. Nat Rev Dis Primers 2019;5:56. - PubMed
-
- Balling M, Nordestgaard BG, Langsted A, Varbo A, Kamstrup PR, Afzal S. Small dense low-density lipoprotein cholesterol predicts atherosclerotic cardiovascular disease in the Copenhagen General Population Study. J Am Coll Cardiol 2020;75:2873–2875. - PubMed
-
- Ginsberg HN, Packard CJ, Chapman MJet al. Triglyceride-rich lipoproteins and their remnants: metabolic insights, role in atherosclerotic cardiovascular disease, and emerging therapeutic strategies—a consensus statement from the European Atherosclerosis Society. Eur Heart J; doi:10.1093/eurheartj/ehab551. Published online ahead of print 2021. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

