The yapsin family of aspartyl proteases regulate glucose homeostasis in Candida glabrata
- PMID: 35051415
- PMCID: PMC8844688
- DOI: 10.1016/j.jbc.2022.101593
The yapsin family of aspartyl proteases regulate glucose homeostasis in Candida glabrata
Abstract
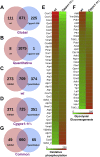
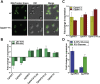
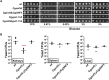
Invasive candidiasis poses a major healthcare threat. The human opportunistic fungal pathogen Candida glabrata, which causes mucosal and deep-seated infections, is armed with distinct virulence attributes, including a family of 11 glycosylphosphatidylinositol-linked aspartyl proteases, CgYapsins. Here, we have profiled total membrane proteomes of the C. glabrata wildtype and 11 proteases-deficient strain, Cgyps1-11Δ, by mass spectrometry analysis and uncovered a novel role for fungal yapsins in glucose sensing and homeostasis. Furthermore, through label-free quantitative membrane proteome analysis, we showed differential abundance of 42% of identified membrane proteins, with electron transport chain and glycolysis proteins displaying lower and higher abundance in Cgyps1-11Δ cells, compared with wildtype cells, respectively. We also demonstrated elevated glucose uptake and upregulation of genes that code for the low-glucose sensor CgSnf3, transcriptional regulators CgMig1 and CgRgt1, and hexose transporter CgHxt2/10 in the Cgyps1-11Δ mutant. We further elucidated a potential underlying mechanism through genetic and transcript measurement analysis under low- and high-glucose conditions and found CgSNF3 deletion to rescue high glucose uptake and attenuated growth of the Cgyps1-11Δ mutant in YPD medium, thereby linking CgYapsins with regulation of the CgSnf3-dependent low-glucose sensing pathway. Last, high ethanol production, diminished mitochondrial membrane potential, and elevated susceptibility to oxidative phosphorylation inhibitors point toward increased fermentative and decreased respiratory metabolism in the Cgyps1-11Δ mutant. Altogether, our findings revealed new possible glucose metabolism-regulatory roles for putative cell surface-associated CgYapsins and advanced our understanding of fungal carbohydrate homeostasis mechanisms.
Keywords: GPI-linked aspartyl proteases; aspartic protease; glucose; glucose sensing and uptake; hexose transporters; high-affinity glucose sensor Snf3; human pathogenic fungi; membrane proteome; proteomics; respiration.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflict of interest with the contents of this article.
Figures









References
-
- Yike I. Fungal proteases and their pathophysiological effects. Mycopathologia. 2011;171:299–323. - PubMed
-
- Rapala-Kozik M., Bochenska O., Zajac D., Karkowska-Kuleta J., Gogol M., Zawrotniak M., Kozik A. Extracellular proteinases of Candida species pathogenic yeasts. Mol. Oral Microbiol. 2018;33:113–124. - PubMed
-
- Gagnon-Arsenault I., Tremblay J., Bourbonnais Y. Fungal yapsins and cell wall: A unique family of aspartic peptidases for a distinctive cellular function. FEMS Yeast Res. 2006;6:966–978. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

