Passive immunization against phosphorylated tau improves features of Huntington's disease pathology
- PMID: 35051614
- PMCID: PMC9077324
- DOI: 10.1016/j.ymthe.2022.01.020
Passive immunization against phosphorylated tau improves features of Huntington's disease pathology
Abstract
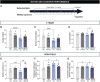
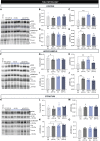
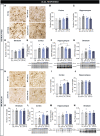
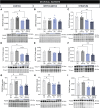
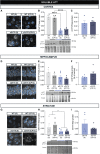
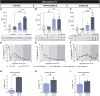
Huntington's disease is classically described as a neurodegenerative disorder of monogenic aetiology. The disease is characterized by an abnormal polyglutamine expansion in the huntingtin gene, which drives the toxicity of the mutated form of the protein. However, accumulation of the microtubule-associated protein tau, which is involved in a number of neurological disorders, has also been observed in patients with Huntington's disease. In order to unravel the contribution of tau hyperphosphorylation to hallmark features of Huntington's disease, we administered weekly intraperitoneal injections of the anti-tau pS202 CP13 monoclonal antibody to zQ175 mice and characterized the resulting behavioral and biochemical changes. After 12 weeks of treatment, motor impairments, cognitive performance and general health were improved in zQ175 mice along with a significant reduction in hippocampal pS202 tau levels. Despite the lack of effect of CP13 on neuronal markers associated with Huntington's disease pathology, tau-targeting enzymes and gliosis, CP13 was shown to directly impact mutant huntingtin aggregation such that brain levels of amyloid fibrils and huntingtin oligomers were decreased, while larger huntingtin protein aggregates were increased. Investigation of CP13 treatment of Huntington's disease patient-derived induced pluripotent stem cells (iPSCs) revealed a reduction in pS202 levels in differentiated cortical neurons and a rescue of neurite length. Collectively, these findings suggest that attenuating tau pathology could mitigate behavioral and molecular hallmarks associated with Huntington's disease.
Keywords: antibody; huntingtin; hyperphosphorylation; iPSC; mHTT; neurodegenerative diseases; pS202 tau; tauopathy; zQ175.
Copyright © 2022 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

