A Review on Secondary Immune Thrombocytopenia in Malaysia
- PMID: 35052202
- PMCID: PMC8774929
- DOI: 10.3390/healthcare10010038
A Review on Secondary Immune Thrombocytopenia in Malaysia
Abstract
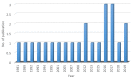
Immune thrombocytopenia (ITP) is an acquired autoimmune disease that occurs in adults and children. In Malaysia, the clinical practice guideline (CPG) for the management of ITP was issued in 2006, which focused almost exclusively on primary ITP (pITP), and only a few secondary ITP (sITP) forms were addressed. All published (twenty-three) sITP articles among children and adults in Malaysia, identified on the academic databases were retrieved. The articles were published between 1981 and 2019, at a rate of 0.62 publications per year. The publications were considered low and mainly focused on rare presentation and followed-up of secondary diseases. This review revealed that sITP in Malaysia is commonly associated with autoimmune diseases (Evan's syndrome, SLE and WAS), malignancy (Kaposi's sarcoma and breast cancer) and infection (dengue haemorrhagic fever, Helicobacter pylori and hepatitis C virus). The relationship between ITP and autoimmune diseases, malignancy and infections raise the question concerning the mechanism involved in these associations. Further studies should be conducted to bridge the current knowledge gap, and the further information is required to update the existing CPG of management of ITP in Malaysia.
Keywords: autoimmune disease; clinical practice guideline; infection; malignancy; secondary immune thrombocytopenia.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
References
-
- Clinical practice guidelines: Management of Immune Thrombocytopenic Purpura. [(accessed on 1 September 2021)]. Available online: http://www.acadmed.org.my/index.cfm?menuid=67.
-
- Rodeghiero F., Stasi R., Gernsheimer T., Michel M., Provan D., Arnold D.M., Bussel J.B., Cines D.B., Chong B.H., Cooper N. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: Report from an international working group. Blood. 2009;113:2386–2393. doi: 10.1182/blood-2008-07-162503. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous