MEK Inhibition in a Newborn with RAF1-Associated Noonan Syndrome Ameliorates Hypertrophic Cardiomyopathy but Is Insufficient to Revert Pulmonary Vascular Disease
- PMID: 35052347
- PMCID: PMC8774485
- DOI: 10.3390/genes13010006
MEK Inhibition in a Newborn with RAF1-Associated Noonan Syndrome Ameliorates Hypertrophic Cardiomyopathy but Is Insufficient to Revert Pulmonary Vascular Disease
Abstract
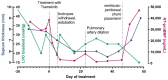
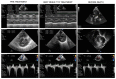
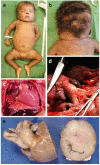
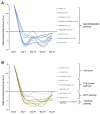
The RAF1:p.Ser257Leu variant is associated with severe Noonan syndrome (NS), progressive hypertrophic cardiomyopathy (HCM), and pulmonary hypertension. Trametinib, a MEK-inhibitor approved for treatment of RAS/MAPK-mutated cancers, is an emerging treatment option for HCM in NS. We report a patient with NS and HCM, treated with Trametinib and documented by global RNA sequencing before and during treatment to define transcriptional effects of MEK-inhibition. A preterm infant with HCM carrying the RAF1:p.Ser257Leu variant, rapidly developed severe congestive heart failure (CHF) unresponsive to standard treatments. Trametinib was introduced (0.022 mg/kg/day) with prompt clinical improvement and subsequent amelioration of HCM at ultrasound. The appearance of pulmonary artery aneurysm and pulmonary hypertension contributed to a rapid worsening after ventriculoperitoneal shunt device placement for posthemorrhagic hydrocephalus: she deceased for untreatable CHF at 3 months of age. Autopsy showed severe obstructive HCM, pulmonary artery dilation, disarrayed pulmonary vascular anatomy consistent with pulmonary capillary hemangiomatosis. Transcriptome across treatment, highlighted robust transcriptional changes induced by MEK-inhibition. Our findings highlight a previously unappreciated connection between pulmonary vascular disease and the severe outcome already reported in patients with RAF1-associated NS. While MEK-inhibition appears a promising therapeutic option for HCM in RASopathies, it appears insufficient to revert pulmonary hypertension.
Keywords: MEK-inhibitor; Noonan syndrome; RASopathies; hypertrophic cardiomyopathy; therapeutics; transcriptomics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Tartaglia M., Kalidas K., Shaw A., Song X., Musat D.L., van der Burgt I., Brunner H.G., Bertola D., Crosby A., Ion A., et al. PTPN11 mutations in Noonan syndrome: Molecular spectrum, genotype-phenotype correlation, and phenotypic heterogeneity. Am. J. Hum. Genet. 2002;70:1555–1563. doi: 10.1086/340847. - DOI - PMC - PubMed
-
- Pandit B., Sarkozy A., Pennacchio L., Carta C., Oishi K., Martinelli S., Pogna E.A., Schackwitz W., Ustaszewska A., Landstrom A., et al. Gain-of-function RAF1 mutations cause Noonan and LEOPARD syndromes with hypertrophic cardiomyopathy. Nat Genet. 2007;39:1007–1012. doi: 10.1038/ng2073. - DOI - PubMed
-
- Motta M., Giancotti A., Mastromoro G., Chandramouli B., Pinna V., Pantaleoni F., Di Giosaffatte N., Petrini S., Mazza T., D’Ambrosio V., et al. Clinical and functional characterization of a novel RASopathy-causing SHOC2 mutation associated with prenatal-onset hypertrophic cardiomyopathy. Hum. Mutat. 2019;40:1046–1056. doi: 10.1002/humu.23767. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

