Transcriptome Analysis Reveals Differentially Expressed Genes That Regulate Biosynthesis of the Active Compounds with Methyl Jasmonate in Rosemary Suspension Cells
- PMID: 35052408
- PMCID: PMC8775320
- DOI: 10.3390/genes13010067
Transcriptome Analysis Reveals Differentially Expressed Genes That Regulate Biosynthesis of the Active Compounds with Methyl Jasmonate in Rosemary Suspension Cells
Abstract
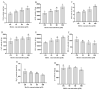
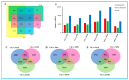
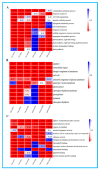
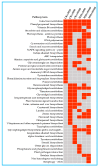
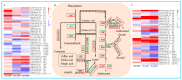
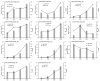
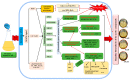
To study the effects of Methyl jasmonates (MeJA) on rosemary suspension cells, the antioxidant enzymes' change of activities under different concentrations of MeJA, including 0 (CK), 10 (M10), 50 (M50) and 100 μM MeJA (M100). The results demonstrated that MeJA treatments increased the activities of phenylalanine ammonla-lyase (PAL), superoxide dismutase (SOD), peroxidase (POD), catalase (CAT) and polyphenol oxidase (PPO) and reduced the contents of hydrogen peroxide (H2O2) and malondialdehyde (MDA), thus accelerating the ROS scavenging. Comparative transcriptome analysis of different concentrations of MeJA showed that a total of 7836, 6797 and 8310 genes were differentially expressed in the comparisons of CKvsM10, CKvsM50, CKvsM100, respectively. The analysis of differentially expressed genes (DEGs) showed phenylpropanoid biosynthesis, vitamin B6, ascorbate and aldarate metabolism-related genes were significantly enriched. The transcripts of flavonoid and terpenoid metabolism pathways and plant hormone signal transduction, especially the jasmonic acid (JA) signal-related genes, were differentially expressed in CKvsM50 and CKvsM100 comparisons. In addition, the transcription factors (TFs), e.g., MYC2, DELLA, MYB111 played a key role in rosemary suspension cells under MeJA treatments. qRT-PCR of eleven DEGs showed a high correlation between the RNA-seq and the qRT-PCR result. Taken together, MeJA alleviated peroxidative damage of the rosemary suspension cells in a wide concentration range via concentration-dependent differential expression patterns. This study provided a transcriptome sequence resource responding to MeJA and a valuable resource for the genetic and genomic studies of the active compounds engineering in rosemary.
Keywords: MeJA; RNA-seq; Rosmarinus officinalis Lour.; antioxidant enzymes; qRT-PCR; suspension cells; transcription factors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Exploring the Effect of Methyl Jasmonate on the Expression of microRNAs Involved in Biosynthesis of Active Compounds of Rosemary Cell Suspension Cultures through RNA-Sequencing.Int J Mol Sci. 2022 Mar 28;23(7):3704. doi: 10.3390/ijms23073704. Int J Mol Sci. 2022. PMID: 35409063 Free PMC article.
-
Methyl jasmonate treatment induces changes in fruit ripening by modifying the expression of several ripening genes in Fragaria chiloensis fruit.Plant Physiol Biochem. 2013 Sep;70:433-44. doi: 10.1016/j.plaphy.2013.06.008. Epub 2013 Jun 21. Plant Physiol Biochem. 2013. PMID: 23835361
-
An integrated proteomic approach to decipher the effect of methyl jasmonate elicitation on the proteome of Silybum marianum L. hairy roots.Plant Physiol Biochem. 2013 Sep;70:115-22. doi: 10.1016/j.plaphy.2013.05.031. Epub 2013 May 31. Plant Physiol Biochem. 2013. PMID: 23771036
-
Methyl Jasmonate: An Alternative for Improving the Quality and Health Properties of Fresh Fruits.Molecules. 2016 May 31;21(6):567. doi: 10.3390/molecules21060567. Molecules. 2016. PMID: 27258240 Free PMC article. Review.
-
MeJA and Post-Harvest Storage of Fruits and Vegetables and Metabolite Synthesis in Plants.Physiol Plant. 2025 May-Jun;177(3):e70304. doi: 10.1111/ppl.70304. Physiol Plant. 2025. PMID: 40457756 Review.
Cited by
-
System network analysis of Rosmarinus officinalis transcriptome and metabolome-Key genes in biosynthesis of secondary metabolites.PLoS One. 2023 Mar 2;18(3):e0282316. doi: 10.1371/journal.pone.0282316. eCollection 2023. PLoS One. 2023. PMID: 36862714 Free PMC article.
-
Eco-friendly approaches to phytochemical production: elicitation and beyond.Nat Prod Bioprospect. 2024 Jan 10;14(1):5. doi: 10.1007/s13659-023-00419-7. Nat Prod Bioprospect. 2024. PMID: 38195902 Free PMC article. Review.
-
MeJA-induced hairy roots in Plumbago auriculata L. by RNA-seq profiling and key synthase provided new insights into the sustainable production of plumbagin and saponins.Front Plant Sci. 2024 Jul 12;15:1411963. doi: 10.3389/fpls.2024.1411963. eCollection 2024. Front Plant Sci. 2024. PMID: 39070915 Free PMC article.
-
Selection and Validation of the Most Suitable Reference Genes for Quantitative Real-Time PCR Normalization in Salvia rosmarinus under In Vitro Conditions.Plants (Basel). 2022 Oct 27;11(21):2878. doi: 10.3390/plants11212878. Plants (Basel). 2022. PMID: 36365331 Free PMC article.
-
Comparative Analysis of Hulless Barley Transcriptomes to Regulatory Effects of Phosphorous Deficiency.Life (Basel). 2024 Jul 19;14(7):904. doi: 10.3390/life14070904. Life (Basel). 2024. PMID: 39063656 Free PMC article.
References
-
- del Baño M.J., Lorente J., Castillo J., Benavente-García O., Marín M.P., Del Río J.A., Ortuño A., Ibarra I. Flavonoid Distribution during the Development of Leaves, Flowers, Stems, and Roots of Rosmarinus officinalis. Postulation of a Biosynthetic Pathway. J. Agric. Food Chem. 2004;52:4987–4992. doi: 10.1021/jf040078p. - DOI - PubMed
-
- da Silva Bomfim N., Nakassugi L.P., Oliveira J.F., Kohiyama C.Y., Mossini S.A., Grespan R., Nerilo S.B., Mallmann C.A., Abreu Filho B.A., Machinski M., Jr. Antifungal activity and inhibition of fumonisin production by Rosmarinus officinalis L. essential oil in Fusarium verticillioides (Sacc.) Nirenberg—ScienceDirect. Food Chem. 2015;166:330–336. doi: 10.1016/j.foodchem.2014.06.019. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

