A New Insight into an Alternative Therapeutic Approach to Restore Redox Homeostasis and Functional Mitochondria in Neurodegenerative Diseases
- PMID: 35052511
- PMCID: PMC8772965
- DOI: 10.3390/antiox11010007
A New Insight into an Alternative Therapeutic Approach to Restore Redox Homeostasis and Functional Mitochondria in Neurodegenerative Diseases
Abstract
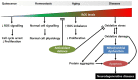
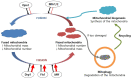
Neurodegenerative diseases are accompanied by oxidative stress and mitochondrial dysfunction, leading to a progressive loss of neuronal cells, formation of protein aggregates, and a decrease in cognitive or motor functions. Mitochondrial dysfunction occurs at the early stage of neurodegenerative diseases. Protein aggregates containing oxidatively damaged biomolecules and other misfolded proteins and neuroinflammation have been identified in animal models and patients with neurodegenerative diseases. A variety of neurodegenerative diseases commonly exhibits decreased activity of antioxidant enzymes, lower amounts of antioxidants, and altered cellular signalling. Although several molecules have been approved clinically, there is no known cure for neurodegenerative diseases, though some drugs are focused on improving mitochondrial function. Mitochondrial dysfunction is caused by oxidative damage and impaired cellular signalling, including that of peroxisome proliferator-activated receptor gamma coactivator 1α. Mitochondrial function can also be modulated by mitochondrial biogenesis and the mitochondrial fusion/fission cycle. Mitochondrial biogenesis is regulated mainly by sirtuin 1, NAD+, AMP-activated protein kinase, mammalian target of rapamycin, and peroxisome proliferator-activated receptor γ. Altered mitochondrial dynamics, such as increased fission proteins and decreased fusion products, are shown in neurodegenerative diseases. Due to the restrictions of a target-based approach, a phenotype-based approach has been performed to find novel proteins or pathways. Alternatively, plasma membrane redox enzymes improve mitochondrial function without the further production of reactive oxygen species. In addition, inducers of antioxidant response elements can be useful to induce a series of detoxifying enzymes. Thus, redox homeostasis and metabolic regulation can be important therapeutic targets for delaying the progression of neurodegenerative diseases.
Keywords: mitochondrial biogenesis; mitochondrial dynamics; mitochondrial dysfunction; neurodegenerative diseases; neuroinflammation; oxidative stress; plasma membrane redox enzymes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

