Hydrogen Peroxide and Amyotrophic Lateral Sclerosis: From Biochemistry to Pathophysiology
- PMID: 35052556
- PMCID: PMC8773294
- DOI: 10.3390/antiox11010052
Hydrogen Peroxide and Amyotrophic Lateral Sclerosis: From Biochemistry to Pathophysiology
Abstract
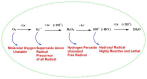
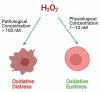
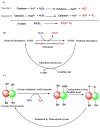
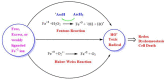
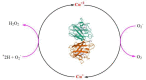
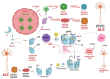
Free radicals are unstable chemical reactive species produced during Redox dyshomeostasis (RDH) inside living cells and are implicated in the pathogenesis of various neurodegenerative diseases. One of the most complicated and life-threatening motor neurodegenerative diseases (MND) is amyotrophic lateral sclerosis (ALS) because of the poor understanding of its pathophysiology and absence of an effective treatment for its cure. During the last 25 years, researchers around the globe have focused their interest on copper/zinc superoxide dismutase (Cu/Zn SOD, SOD1) protein after the landmark discovery of mutant SOD1 (mSOD1) gene as a risk factor for ALS. Substantial evidence suggests that toxic gain of function due to redox disturbance caused by reactive oxygen species (ROS) changes the biophysical properties of native SOD1 protein thus, instigating its fibrillization and misfolding. These abnormal misfolding aggregates or inclusions of SOD1 play a role in the pathogenesis of both forms of ALS, i.e., Sporadic ALS (sALS) and familial ALS (fALS). However, what leads to a decrease in the stability and misfolding of SOD1 is still in question and our scientific knowledge is scarce. A large number of studies have been conducted in this area to explore the biochemical mechanistic pathway of SOD1 aggregation. Several studies, over the past two decades, have shown that the SOD1-catalyzed biochemical reaction product hydrogen peroxide (H2O2) at a pathological concentration act as a substrate to trigger the misfolding trajectories and toxicity of SOD1 in the pathogenesis of ALS. These toxic aggregates of SOD1 also cause aberrant localization of TAR-DNA binding protein 43 (TDP-43), which is characteristic of neuronal cytoplasmic inclusions (NCI) found in ALS. Here in this review, we present the evidence implicating the pivotal role of H2O2 in modulating the toxicity of SOD1 in the pathophysiology of the incurable and highly complex disease ALS. Also, highlighting the role of H2O2 in ALS, we believe will encourage scientists to target pathological concentrations of H2O2 thereby halting the misfolding of SOD1.
Keywords: TAR-DNA binding protein 43; aggregation; amyotrophic lateral sclerosis; fibrilization; hydrogen peroxide; misfolding; motor neurodegenerative disease; mutant superoxide dismutase 1; neuronal cytoplasmic inclusions; reactive oxygen species; redox dyshomeostasis; superoxide dismutase 1.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
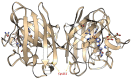
References
-
- Carmo C., Naia L., Lopes C., Rego A.C. Mitochondrial Dysfunction in Huntington’s Disease. Adv. Exp. Med. Biol. 2018;1049:59–83. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

