SKP-SC-EVs Mitigate Denervated Muscle Atrophy by Inhibiting Oxidative Stress and Inflammation and Improving Microcirculation
- PMID: 35052570
- PMCID: PMC8772917
- DOI: 10.3390/antiox11010066
SKP-SC-EVs Mitigate Denervated Muscle Atrophy by Inhibiting Oxidative Stress and Inflammation and Improving Microcirculation
Abstract
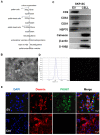
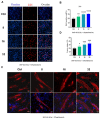
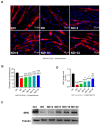
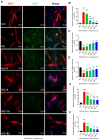
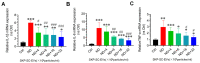
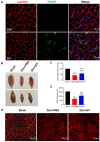
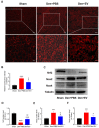
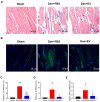
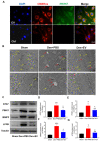
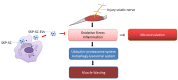
Denervated muscle atrophy is a common clinical disease that has no effective treatments. Our previous studies have found that oxidative stress and inflammation play an important role in the process of denervated muscle atrophy. Extracellular vesicles derived from skin precursor-derived Schwann cells (SKP-SC-EVs) contain a large amount of antioxidants and anti-inflammatory factors. This study explored whether SKP-SC-EVs alleviate denervated muscle atrophy by inhibiting oxidative stress and inflammation. In vitro studies have found that SKP-SC-EVs can be internalized and caught by myoblasts to promote the proliferation and differentiation of myoblasts. Nutrient deprivation can cause myotube atrophy, accompanied by oxidative stress and inflammation. However, SKP-SC-EVs can inhibit oxidative stress and inflammation caused by nutritional deprivation and subsequently relieve myotube atrophy. Moreover, there is a remarkable dose-effect relationship. In vivo studies have found that SKP-SC-EVs can significantly inhibit a denervation-induced decrease in the wet weight ratio and myofiber cross-sectional area of target muscles. Furthermore, SKP-SC-EVs can dramatically inhibit highly expressed Muscle RING Finger 1 and Muscle Atrophy F-box in target muscles under denervation and reduce the degradation of the myotube heavy chain. SKP-SC-EVs may reduce mitochondrial vacuolar degeneration and autophagy in denervated muscles by inhibiting autophagy-related proteins (i.e., PINK1, BNIP3, LC3B, and ATG7). Moreover, SKP-SC-EVs may improve microvessels and blood perfusion in denervated skeletal muscles by enhancing the proliferation of vascular endothelial cells. SKP-SC-EVs can also significantly inhibit the production of reactive oxygen species (ROS) in target muscles after denervation, which indicates that SKP-SC-EVs elicit their role by upregulating Nrf2 and downregulating ROS production-related factors (Nox2 and Nox4). In addition, SKP-SC-EVs can significantly reduce the levels of interleukin 1β, interleukin-6, and tumor necrosis factor α in target muscles. To conclude, SKP-SC-EVs may alleviate the decrease of target muscle blood perfusion and passivate the activities of ubiquitin-proteasome and autophagy-lysosome systems by inhibiting oxidative stress and inflammatory response, then reduce skeletal muscle atrophy caused by denervation. This study not only enriches the molecular regulation mechanism of denervated muscle atrophy, but also provides a scientific basis for SKP-SC-EVs as a protective drug to prevent and treat muscle atrophy.
Keywords: SKP-SC-EVs; denervated muscle atrophy; inflammation; microcirculation; oxidative stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Qiu J., Fang Q., Xu T., Wu C., Xu L., Wang L., Yang X., Yu S., Zhang Q., Ding F., et al. Mechanistic role of reactive oxygen species and therapeutic potential of antioxidants in denervation- or fasting-induced skeletal muscle atrophy. Front. Physiol. 2018;9:215. doi: 10.3389/fphys.2018.00215. - DOI - PMC - PubMed
-
- Anderson L.J., Liu H., Garcia J.M. Sex differences in muscle wasting. Ad. Exp. Med. Biol. 2017;1043:153–197. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

