The Response to Oxidative Damage Correlates with Driver Mutations and Clinical Outcome in Patients with Myelofibrosis
- PMID: 35052617
- PMCID: PMC8772737
- DOI: 10.3390/antiox11010113
The Response to Oxidative Damage Correlates with Driver Mutations and Clinical Outcome in Patients with Myelofibrosis
Abstract
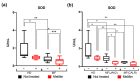
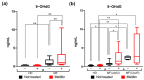
Myelofibrosis (MF) is the Philadelphia-negative myeloproliferative neoplasm characterized by the worst prognosis and no response to conventional therapy. Driver mutations in JAK2 and CALR impact on JAK-STAT pathway activation but also on the production of reactive oxygen species (ROS). ROS play a pivotal role in inflammation-induced oxidative damage to cellular components including DNA, therefore leading to greater genomic instability and promoting cell transformation. In order to unveil the role of driver mutations in oxidative stress, we assessed ROS levels in CD34+ hematopoietic stem/progenitor cells of MF patients. Our results demonstrated that ROS production in CD34+ cells from CALR-mutated MF patients is far greater compared with patients harboring JAK2 mutation, and this leads to increased oxidative DNA damage. Moreover, CALR-mutant cells show less superoxide dismutase (SOD) antioxidant activity than JAK2-mutated ones. Here, we show that high plasma levels of total antioxidant capacity (TAC) correlate with detrimental clinical features, such as high levels of lactate dehydrogenase (LDH) and circulating CD34+ cells. Moreover, in JAK2-mutated patients, high plasma level of TAC is also associated with a poor overall survival (OS), and multivariate analysis demonstrated that high TAC classification is an independent prognostic factor allowing the identification of patients with inferior OS in both DIPSS lowest and highest categories. Altogether, our data suggest that a different capability to respond to oxidative stress can be one of the mechanisms underlying disease progression of myelofibrosis.
Keywords: 8-hydroxy-2′-deoxy-guanosine; calreticulin; janus kinase 2; myelofibrosis; oxidative stress; reactive oxygen species; superoxide dismutase; total antioxidant capacity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Sies H. Biochemistry of Oxidative Stress. Angew. Chem. Int. Ed. Engl. 1986;25:1058–1071. doi: 10.1002/anie.198610581. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

