Effect of 5-Azacitidine Treatment on Redox Status and Inflammatory Condition in MDS Patients
- PMID: 35052643
- PMCID: PMC8773071
- DOI: 10.3390/antiox11010139
Effect of 5-Azacitidine Treatment on Redox Status and Inflammatory Condition in MDS Patients
Abstract
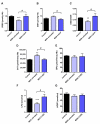
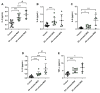
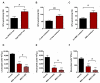
This study focused on the impact of the treatment with the hypomethylating agent 5-azacitidine on the redox status and inflammation in 24 MDS patients. Clinical and genetic features of MDS patients were recorded, and peripheral blood samples were used to determine the activity of the endogenous antioxidant defense system (superoxide dismutase, SOD; catalase, CAT; glutathion peroxidase, GPx; and reductase, GRd, activities), markers of oxidative damage (lipid peroxidation, LPO, and advanced oxidation protein products, AOPP). Moreover, pro-inflammatory cytokines and plasma nitrite plus nitrate levels as markers of inflammation, as well as CoQ10 plasma levels, were also measured. Globally, MDS patients showed less redox status in terms of a reduction in the GSSG/GSH ratio and in the LPO levels, as well as increased CAT activity compared with healthy subjects, with no changes in SOD, GPx, and GRd activities, or AOPP levels. When analyzing the evolution from early to advanced stages of the disease, we found that the GPx activity, GSSG/GSH ratio, LPO, and AOPP increased, with a reduction in CAT. GPx changes were related to the presence of risk factors such as high-risk IPSS-R or mutational score. Moreover, there was an increase in IL-2, IL-6, IL-8, and TNF-α plasma levels, with a further increase of IL-2 and IL-10 from early to advanced stages of the disease. However, we did not observe any association between inflammation and oxidative stress. Finally, 5-azacitidine treatment generated oxidative stress in MDS patients, without affecting inflammation levels, suggesting that oxidative status and inflammation are two independent processes.
Keywords: 5-azacitidine; cytokines; inflammation; myelodysplastic syndrome (MDS); oxidative stress; somatic alterations.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

