The Trypanosoma brucei-Derived Ketoacids, Indole Pyruvate and Hydroxyphenylpyruvate, Induce HO-1 Expression and Suppress Inflammatory Responses in Human Dendritic Cells
- PMID: 35052669
- PMCID: PMC8772738
- DOI: 10.3390/antiox11010164
The Trypanosoma brucei-Derived Ketoacids, Indole Pyruvate and Hydroxyphenylpyruvate, Induce HO-1 Expression and Suppress Inflammatory Responses in Human Dendritic Cells
Abstract
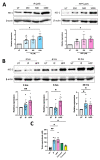
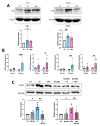
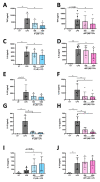
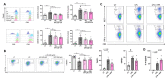
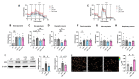
The extracellular parasite and causative agent of African sleeping sickness Trypanosoma brucei (T. brucei) has evolved a number of strategies to avoid immune detection in the host. One recently described mechanism involves the conversion of host-derived amino acids to aromatic ketoacids, which are detected at relatively high concentrations in the bloodstream of infected individuals. These ketoacids have been shown to directly suppress inflammatory responses in murine immune cells, as well as acting as potent inducers of the stress response enzyme, heme oxygenase 1 (HO-1), which has proven anti-inflammatory properties. The aim of this study was to investigate the immunomodulatory properties of the T. brucei-derived ketoacids in primary human immune cells and further examine their potential as a therapy for inflammatory diseases. We report that the T. brucei-derived ketoacids, indole pyruvate (IP) and hydroxyphenylpyruvate (HPP), induce HO-1 expression through Nrf2 activation in human dendritic cells (DC). They also limit DC maturation and suppress the production of pro-inflammatory cytokines, which, in turn, leads to a reduced capacity to differentiate adaptive CD4+ T cells. Furthermore, the ketoacids are capable of modulating DC cellular metabolism and suppressing the inflammatory profile of cells isolated from patients with inflammatory bowel disease. This study therefore not only provides further evidence of the immune-evasion mechanisms employed by T. brucei, but also supports further exploration of this new class of HO-1 inducers as potential therapeutics for the treatment of inflammatory conditions.
Keywords: Trypanosoma brucei; anti-inflammatory therapies; aromatic ketoacids; dendritic cells; heme oxygenase 1; immunomodulation; inflammatory bowel disease.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Marciano D., Llorente C., Maugeri D.A., de la Fuente C., Opperdoes F., Cazzulo J.J., Nowicki C. Biochemical characterization of stage-specific isoforms of aspartate aminotransferases from Trypanosoma cruzi and Trypanosoma brucei. Mol. Biochem. Parasitol. 2008;161:12–20. doi: 10.1016/j.molbiopara.2008.05.005. - DOI - PubMed
-
- McGettrick A.F., Corcoran S.E., Barry P.J.G., McFarland J., Crès C., Curtis A.M., Franklin E., Corr S.C., Mok K.H., Cummins E.P., et al. Trypanosoma brucei metabolite indolepyruvate decreases HIF-1α and glycolysis in macrophages as a mechanism of innate immune evasion. Proc. Natl. Acad. Sci. USA. 2016;113:E7778–E7787. doi: 10.1073/pnas.1608221113. - DOI - PMC - PubMed
-
- Campbell N.K., Williams D.G., Fitzgerald H.K., Barry P.J., Cunningham C.C., Nolan D.P., Dunne A. Trypanosoma brucei Secreted Aromatic Ketoacids Activate the Nrf2/HO-1 Pathway and Suppress Pro-inflammatory Responses in Primary Murine Glia and Macrophages. Front. Immunol. 2019;10:2137. doi: 10.3389/fimmu.2019.02137. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

