Characterization of the Gut Microbiota in Individuals with Overweight or Obesity during a Real-World Weight Loss Dietary Program: A Focus on the Bacteroides 2 Enterotype
- PMID: 35052696
- PMCID: PMC8772804
- DOI: 10.3390/biomedicines10010016
Characterization of the Gut Microbiota in Individuals with Overweight or Obesity during a Real-World Weight Loss Dietary Program: A Focus on the Bacteroides 2 Enterotype
Abstract
Background: Dietary intervention is a cornerstone of weight loss therapies. In obesity, a dysbiotic gut microbiota (GM) is characterized by high levels of Bacteroides lineages and low diversity. We examined the GM composition changes, including the Bacteroides 2 enterotype (Bact2), in a real-world weight loss study in subjects following a high-protein hypocaloric diet with or without a live microorganisms (LMP) supplement.
Method: 263 volunteers were part of this real-world weight loss program. The first phase was a high-protein low-carbohydrate calorie restriction diet with or without LMP supplements. Fecal samples were obtained at baseline and after 10% weight loss for 163 subjects. Metagenomic profiling was obtained by shotgun sequencing.
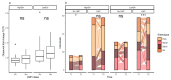
Results: At baseline, the Bact2 enterotype was more prevalent in subjects with aggravated obesity and metabolic alterations. After weight loss, diversity increased and Bact2 prevalence decreased in subjects with lower GM diversity at baseline, notably in LMP consumers. Significant increases in Akkermansia muciniphila and Parabacteroides distasonis and significant decreases of Eubacterium rectale, Streptococcus thermophilus and Bifidobacterial lineages were observed after weight loss.
Conclusions: Baseline microbiome composition is associated with differential changes in GM diversity and Bact2 enterotype prevalence after weight loss. Examining these signatures could drive future personalized nutrition efforts towards more favorable microbiome compositions.
Keywords: Bacteroides 2 enterotype; gut microbiota; nanopore technology; obesity; real-world dietary intervention; weight loss.
Conflict of interest statement
T.D.S., N.G. and E.B. work at Integrative Phenomics (Paris, France). K.C. has received grants from Danone Research (Palaiseau, France), Ysopia (Bordeaux, France), and CONFO therapeutics (Gent, Belgium); however, for work not associated with this study.
Figures




References
-
- World Health Organization (WHO) [(accessed on 10 October 2021)]. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight.
LinkOut - more resources
Full Text Sources

