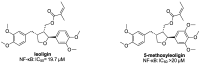Investigation of Leoligin Derivatives as NF-κΒ Inhibitory Agents
- PMID: 35052742
- PMCID: PMC8773117
- DOI: 10.3390/biomedicines10010062
Investigation of Leoligin Derivatives as NF-κΒ Inhibitory Agents
Abstract
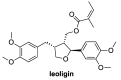
The transcription factor NF-κB is an essential mediator of inflammation; thus, the identification of compounds that interfere with the NF-κB signaling pathway is an important topic. The natural products leoligin and 5-methoxyleoligin have served as a starting point for the development of NF-κB inhibitors. Using our modular total synthesis method of leoligin, modifications at two positions were undertaken and the effects of these modifications on the biological activity were investigated. The first modification concerned the ester functionality, where it was found that variations in this position have a significant influence, with bulky esters lacking Michael-acceptor properties being favored. Additionally, the substituents on the aryl group in position 2 of the tetrahydrofuran scaffold can vary to some extent, where it was found that a 3,4-dimethoxy and a 4-fluoro substitution pattern show comparable inhibitory efficiency.
Keywords: NF-κB inhibition; inflammation; lignans; natural product synthesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Reisinger U., Schwaiger S., Zeller I., Messner B., Stigler R., Wiedemann D., Mayr T., Seger C., Schachner T., Dirsch V.M., et al. Leoligin, the major lignan from Edelweiss, inhibits intimal hyperplasia of venous bypass grafts. Cardiovasc. Res. 2009;82:542–549. doi: 10.1093/cvr/cvp059. - DOI - PMC - PubMed
-
- Weber C., Erl W., Pietsch A., Ströbel M., Ziegler-Heitbrock H.W., Weber P.C. Antioxidants inhibit monocyte adhesion by suppressing nuclear factor-kappa B mobilization and induction of vascular cell adhesion molecule-1 in endothelial cells stimulated to generate radicals. Arter. Thromb. J. Vasc. Biol. 1994;14:1665–1673. doi: 10.1161/01.ATV.14.10.1665. - DOI - PubMed
-
- Linder T., Geyrhofer S., Papaplioura E., Wang L., Atanasov A.G., Stuppner H., Dirsch V.M., Schnürch M., Mihovilovic M.D. Design and Synthesis of a Compound Library Exploiting 5-Methoxyleoligin as Potential Cholesterol Efflux Promoter. Molecules. 2020;25:662. doi: 10.3390/molecules25030662. - DOI - PMC - PubMed
-
- Linder T., Liu R., Atanasov A.G., Li Y., Geyrhofer S., Schwaiger S., Stuppner H., Schnürch M., Dirsch V.M., Mihovilovic M.D. Leoligin-inspired synthetic lignans with selectivity for cell-type and bioactivity relevant for cardiovascular disease. Chem. Sci. 2019;10:5815–5820. doi: 10.1039/C9SC00446G. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources