Effect of Cephalosporin Treatment on the Microbiota and Antibiotic Resistance Genes in Feces of Dairy Cows with Clinical Mastitis
- PMID: 35052994
- PMCID: PMC8773067
- DOI: 10.3390/antibiotics11010117
Effect of Cephalosporin Treatment on the Microbiota and Antibiotic Resistance Genes in Feces of Dairy Cows with Clinical Mastitis
Abstract
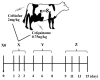
Antibiotics are frequently used to treat dairy cows with mastitis. However, the potential effects of β-lactam antibiotics, such as cephalosporins, on the fecal microbiome is unknown. The objective was to investigate the effects of ceftiofur and cefquinome on the fecal microbiota and antibiotic resistance genes of dairy cows with mastitis. The fecal samples were collected from 8 dairy cows at the following periods: the start day (Day 0), medication (Days 1, 2, and 3), withdrawal (Days 4, 6, 7, and 8), and recovery (Days 9, 11, 13, and 15). 16S rRNA gene sequencing was applied to explore the changes in microbiota, and qPCR was used to investigate the antibiotic resistance genes. The cephalosporin treatment significantly decreased the microbial diversity and richness, indicated by the decreased Shannon and Chao 1 indexes, respectively (p < 0.05). The relative abundance of Bacteroides, Bacteroidaceae, Bacteroidales, and Bacteroidia increased, and the relative abundance of Clostridia, Clostridiales, Ethanoligenens, and Clostridium IV decreased at the withdrawal period. The cephalosporin treatment increased the relative abundance of β-lactam resistance genes (blaTEM and cfxA) at the withdrawal period (p < 0.05). In conclusion, the cephalosporin treatment decreased the microbial diversity and richness at the medication period, and increased the relative abundance of two β-lactam resistance genes at the withdrawal period.
Keywords: antibiotic resistance genes; cephalosporin; dairy cow mastitis; fecal microbiota.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures






References
-
- Turk R., Piras C., Kovai M., Samardžija M., Ahmed H., Canio M.D., Urbani A., Meštrić Z.F., Soggiu A., Bonizzi L., et al. Proteomics of inflammatory and oxidative stress response in cows with subclinical and clinical mastitis. J. Proteom. 2012;75:4412–4428. doi: 10.1016/j.jprot.2012.05.021. - DOI - PubMed
Grants and funding
- CAAS-ZDXT2019004/Scientific Research Project for Major Achievements of the Agricultural Science and Technology Innovation Program (ASTIP)
- ASTIP-IAS12/Agricultural Science and Technology Innovation Program
- China Agriculture Research System of MOF and MARA/China Agriculture Research System of MOF and MARA
- GJFP2019027/Project of Risk Assessment on Raw Milk
LinkOut - more resources
Full Text Sources

